The Atomic Theory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Alternate Graphical Representation of Periodic Table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt
An Alternate Graphical Representation of Periodic table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt. Ltd, Hyderabad, India. [email protected] Abstract Periodic table of chemical elements symbolizes an elegant graphical representation of symmetry at atomic level and provides an overview on arrangement of electrons. It started merely as tabular representation of chemical elements, later got strengthened with quantum mechanical description of atomic structure and recent studies have revealed that periodic table can be formulated using SO(4,2) SU(2) group. IUPAC, the governing body in Chemistry, doesn‟t approve any periodic table as a standard periodic table. The only specific recommendation provided by IUPAC is that the periodic table should follow the 1 to 18 group numbering. In this technical paper, we describe a new graphical representation of periodic table, referred as „Circular form of Periodic table‟. The advantages of circular form of periodic table over other representations are discussed along with a brief discussion on history of periodic tables. 1. Introduction The profoundness of inherent symmetry in nature can be seen at different depths of atomic scales. Periodic table symbolizes one such elegant symmetry existing within the atomic structure of chemical elements. This so called „symmetry‟ within the atomic structures has been widely studied from different prospects and over the last hundreds years more than 700 different graphical representations of Periodic tables have emerged [1]. Each graphical representation of chemical elements attempted to portray certain symmetries in form of columns, rows, spirals, dimensions etc. Out of all the graphical representations, the rectangular form of periodic table (also referred as Long form of periodic table or Modern periodic table) has gained wide acceptance. -

Actinide Ground-State Properties-Theoretical Predictions
Actinide Ground-State Properties Theoretical predictions John M. Wills and Olle Eriksson electron-electron correlations—the electronic energy of the ground state of or nearly fifty years, the actinides interactions among the 5f electrons and solids, molecules, and atoms as a func- defied the efforts of solid-state between them and other electrons—are tional of electron density. The DFT Ftheorists to understand their expected to affect the bonding. prescription has had such a profound properties. These metals are among Low-symmetry crystal structures, impact on basic research in both the most complex of the long-lived relativistic effects, and electron- chemistry and solid-state physics that elements, and in the solid state, they electron correlations are very difficult Walter Kohn, its main inventor, was display some of the most unusual to treat in traditional electronic- one of the recipients of the 1998 behaviors of any series in the periodic structure calculations of metals and, Nobel Prize in Chemistry. table. Very low melting temperatures, until the last decade, were outside the In general, it is not possible to apply large anisotropic thermal-expansion realm of computational ability. And DFT without some approximation. coefficients, very low symmetry crystal yet, it is essential to treat these effects But many man-years of intense research structures, many solid-to-solid phase properly in order to understand the have yielded reliable approximate transitions—the list is daunting. Where physics of the actinides. Electron- expressions for the total energy in does one begin to put together an electron correlations are important in which all terms, except for a single- understanding of these elements? determining the degree to which 5f particle kinetic-energy term, can be In the last 10 years, together with electrons are localized at lattice sites. -

Polymer Exemption Guidance Manual POLYMER EXEMPTION GUIDANCE MANUAL
United States Office of Pollution EPA 744-B-97-001 Environmental Protection Prevention and Toxics June 1997 Agency (7406) Polymer Exemption Guidance Manual POLYMER EXEMPTION GUIDANCE MANUAL 5/22/97 A technical manual to accompany, but not supersede the "Premanufacture Notification Exemptions; Revisions of Exemptions for Polymers; Final Rule" found at 40 CFR Part 723, (60) FR 16316-16336, published Wednesday, March 29, 1995 Environmental Protection Agency Office of Pollution Prevention and Toxics 401 M St., SW., Washington, DC 20460-0001 Copies of this document are available through the TSCA Assistance Information Service at (202) 554-1404 or by faxing requests to (202) 554-5603. TABLE OF CONTENTS LIST OF EQUATIONS............................ ii LIST OF FIGURES............................. ii LIST OF TABLES ............................. ii 1. INTRODUCTION ............................ 1 2. HISTORY............................... 2 3. DEFINITIONS............................. 3 4. ELIGIBILITY REQUIREMENTS ...................... 4 4.1. MEETING THE DEFINITION OF A POLYMER AT 40 CFR §723.250(b)... 5 4.2. SUBSTANCES EXCLUDED FROM THE EXEMPTION AT 40 CFR §723.250(d) . 7 4.2.1. EXCLUSIONS FOR CATIONIC AND POTENTIALLY CATIONIC POLYMERS ....................... 8 4.2.1.1. CATIONIC POLYMERS NOT EXCLUDED FROM EXEMPTION 8 4.2.2. EXCLUSIONS FOR ELEMENTAL CRITERIA........... 9 4.2.3. EXCLUSIONS FOR DEGRADABLE OR UNSTABLE POLYMERS .... 9 4.2.4. EXCLUSIONS BY REACTANTS................ 9 4.2.5. EXCLUSIONS FOR WATER-ABSORBING POLYMERS........ 10 4.3. CATEGORIES WHICH ARE NO LONGER EXCLUDED FROM EXEMPTION .... 10 4.4. MEETING EXEMPTION CRITERIA AT 40 CFR §723.250(e) ....... 10 4.4.1. THE (e)(1) EXEMPTION CRITERIA............. 10 4.4.1.1. LOW-CONCERN FUNCTIONAL GROUPS AND THE (e)(1) EXEMPTION................. -

Chapter 12: Phenomena
Chapter 12: Phenomena Phenomena: Different wavelengths of electromagnetic radiation were directed onto two different metal sample (see picture). Scientists then recorded if any particles were ejected and if so what type of particles as well as the speed of the particle. What patterns do you see in the results that were collected? K Wavelength of Light Where Particles Ejected Speed of Exp. Intensity Directed at Sample Ejected Particle Ejected Particle -7 - 4 푚 1 5.4×10 m Medium Yes e 4.9×10 푠 -8 - 6 푚 2 3.3×10 m High Yes e 3.5×10 푠 3 2.0 m High No N/A N/A 4 3.3×10-8 m Low Yes e- 3.5×106 푚 Electromagnetic 푠 Radiation 5 2.0 m Medium No N/A N/A Ejected Particle -12 - 8 푚 6 6.1×10 m High Yes e 2.7×10 푠 7 5.5×104 m High No N/A N/A Fe Wavelength of Light Where Particles Ejected Speed of Exp. Intensity Directed at Sample Ejected Particle Ejected Particle 1 5.4×10-7 m Medium No N/A N/A -11 - 8 푚 2 3.4×10 m High Yes e 1.1×10 푠 3 3.9×103 m Medium No N/A N/A -8 - 6 푚 4 2.4×10 m High Yes e 4.1×10 푠 5 3.9×103 m Low No N/A N/A -7 - 5 푚 6 2.6×10 m Low Yes e 1.1×10 푠 -11 - 8 푚 7 3.4×10 m Low Yes e 1.1×10 푠 Chapter 12: Quantum Mechanics and Atomic Theory Chapter 12: Quantum Mechanics and Atomic Theory o Electromagnetic Radiation o Quantum Theory o Particle in a Box Big Idea: The structure of atoms o The Hydrogen Atom must be explained o Quantum Numbers using quantum o Orbitals mechanics, a theory in o Many-Electron Atoms which the properties of particles and waves o Periodic Trends merge together. -

Introduction to Chemistry
Introduction to Chemistry Author: Tracy Poulsen Digital Proofer Supported by CK-12 Foundation CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook Introduction to Chem... materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based Authored by Tracy Poulsen collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and 8.5" x 11.0" (21.59 x 27.94 cm) distribution of high-quality educational content that will serve both as core text as well as provide Black & White on White paper an adaptive environment for learning. 250 pages ISBN-13: 9781478298601 Copyright © 2010, CK-12 Foundation, www.ck12.org ISBN-10: 147829860X Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made Please carefully review your Digital Proof download for formatting, available to Users in accordance with the Creative Commons Attribution/Non-Commercial/Share grammar, and design issues that may need to be corrected. Alike 3.0 Unported (CC-by-NC-SA) License (http://creativecommons.org/licenses/by-nc- sa/3.0/), as amended and updated by Creative Commons from time to time (the “CC License”), We recommend that you review your book three times, with each time focusing on a different aspect. which is incorporated herein by this reference. Specific details can be found at http://about.ck12.org/terms. Check the format, including headers, footers, page 1 numbers, spacing, table of contents, and index. 2 Review any images or graphics and captions if applicable. -
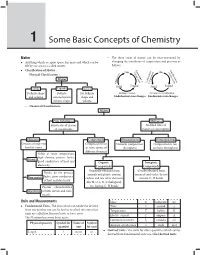
1 Some Basic Concepts of Chemistry
Some Basic Concepts of Chemistry 1 1 Some Basic Concepts of Chemistry Matter – The three states of matter can be inter-converted by Anything which occupies space, has mass and which can be changing the conditions of temperature and pressure as felt by our senses is called matter. follows : Ev Liq Classification of Matter : Co GasGVa ap as uefacti n po orat tion ndensa – tion Physical Classification : io riza at io on n o tion tion Matter lim ndensa or r Deposi Co Sub or Solid Liquid Solid Liquid Solids Liquids Gases Definite shape Definite No definite Melting or Fusion Freezing or Crystallization and volume volume but no shape and Endothermic state changes Exothermic state changes definite shape volume – Chemical Classification : Matter Pure Substances Mixtures Fixed ratio of masses No fixed ratio of of constituents masses of constituents Elements Compounds Homogeneous Heterogeneous Consists of only one Composed of two Uniform composition Composition is not kind of atoms or more atoms of throughout uniform throughout different elements Solids at room temperature, high density, possess lustre, Metals good conductors of heat and Organic Inorganic electricity Compounds Compounds Originally obtained from Usually obtained from Brittle, do not possess animals and plants, contain minerals and rocks, do not lustre, poor conductors Non-metals carbon and few other elements contain C–H bonds of heat and electricity like H, O, S, N, X (halogens), Possess characteristics etc. having C–H bonds Metalloids of both metals and non- metals Units and Measurements Mass m kilogram kg Fundamental Units : The units which can neither be derived Time t second s from one another nor can be further resolved into any other Temperature T kelvin K units are called fundamental units or basic units. -

Atomic Physicsphysics AAA Powerpointpowerpointpowerpoint Presentationpresentationpresentation Bybyby Paulpaulpaul E.E.E
ChapterChapter 38C38C -- AtomicAtomic PhysicsPhysics AAA PowerPointPowerPointPowerPoint PresentationPresentationPresentation bybyby PaulPaulPaul E.E.E. Tippens,Tippens,Tippens, ProfessorProfessorProfessor ofofof PhysicsPhysicsPhysics SouthernSouthernSouthern PolytechnicPolytechnicPolytechnic StateStateState UniversityUniversityUniversity © 2007 Objectives:Objectives: AfterAfter completingcompleting thisthis module,module, youyou shouldshould bebe ableable to:to: •• DiscussDiscuss thethe earlyearly modelsmodels ofof thethe atomatom leadingleading toto thethe BohrBohr theorytheory ofof thethe atom.atom. •• DemonstrateDemonstrate youryour understandingunderstanding ofof emissionemission andand absorptionabsorption spectraspectra andand predictpredict thethe wavelengthswavelengths oror frequenciesfrequencies ofof thethe BalmerBalmer,, LymanLyman,, andand PashenPashen spectralspectral series.series. •• CalculateCalculate thethe energyenergy emittedemitted oror absorbedabsorbed byby thethe hydrogenhydrogen atomatom whenwhen thethe electronelectron movesmoves toto aa higherhigher oror lowerlower energyenergy level.level. PropertiesProperties ofof AtomsAtoms ••• AtomsAtomsAtoms areareare stablestablestable andandand electricallyelectricallyelectrically neutral.neutral.neutral. ••• AtomsAtomsAtoms havehavehave chemicalchemicalchemical propertiespropertiesproperties whichwhichwhich allowallowallow themthemthem tototo combinecombinecombine withwithwith otherotherother atoms.atoms.atoms. ••• AtomsAtomsAtoms emitemitemit andandand absorbabsorbabsorb -

Basic Concepts and Laws of Chemistry. Guidelines and Objectives for Self-Study Courses for Students in All Specialties / O.Y
THE MINISTRY OF EDUCATION AND SCIENCE OF UKRAINE STATE HIGHER EDUCATIONAL INSTITUTION «NATIONAL MINING UNIVERSITY» O.Y. Svietkina, O.B Netyaga, G.V Tarasova BASIC CONCEPTS AND LAWS OF CHEMISTRY. Guidelines and objectives for self-study courses for students in all specialties Dnipro 2016 THE MINISTRY OF EDUCATION AND SCIENCE OF UKRAINE STATE HIGHER EDUCATIONAL INSTITUTION «NATIONAL MINING UNIVERSITY» FACULTY OF GEOLOGICAL PROSPECTING Departament of Chemestry O. Y. Svietkina, O.B Netyaga, G.V Tarasova BASIC CONCEPTS AND LAWS OF CHEMISTRY. Guidelines and objectives for self-study courses for students in all specialties Dnipro NMU 2016 Svietkina O. Y. Basic concepts and laws of chemistry. Guidelines and objectives for self-study courses for students in all specialties / O.Y. Svietkina, O.B. Netyaga, G.V. Tarasova; Ministry of eduk. and sien of Ukrain, Nation. min. univer. – D . : NMU, 2016. – 20 p. Автори: О.Ю. Свєткіна, проф., д-р техн. наук (передмова, розділ 2); О.Б. Нетяга, старш. викл. (розділ 1); Г.В. Тарасова, асист. (розділ 2). Затверджено методичною комісією з галузі знань 10. Природничі науки за поданням кафедри хімії (протокол № 3 від 08.11.2016). The theoretical themes of "Basic concepts and laws of chemistry", are examples of solving common tasks, in order to consolidate the material there are presented self-study tasks for solving. Розглянуто теоретичні положення теми «Основні поняття й закони хімії», наведено приклади розв’язку типових задач з метою закріплення матеріалу, подано задачі для самостійного розв’язування. Відповідальна за випуск завідувач кафедри хімії, д-р техн. наук, проф. О.Ю. Свєткіна. Introduction Chemistry studies such form of substance motion which assumes qualitative change of matters i.e. -

(I) Determination of the Equivalent Weight and Pka of an Organic Acid
Experiment 4 (i) Determination of the Equivalent Weight and pKa of an Organic Acid Discussion This experiment is an example of a common research procedure. Chemists often use two or more analytical techniques to study the same system. These experiments can give complementary qualitative and quantitative information concerning an unknown substance. I. Titration of Acids and Bases in Aqueous Solutions The almost instantaneous reaction between acids and bases in aqueous solution produce changes in pH which one can monitor. Two techniques are useful for detecting the equivalence point: (1) colorimetry, using an acid-base color indicator - a dye which undergoes a sharp change in color in a region of pH covering the equivalence point and (2) potentiometry, using a potentiometer (pH meter) to record the sharp change at the equivalence point in the potential difference between an electrode (usually a glass electrode) and the solution whose pH is undergoing change as a result of the addition of acid or base. For example, in the case of the titration of a weak monoprotic acid HA using sodium hydroxide solution we may write: + - NaOH + HA → Na + A + H2O (4.1) Applying the law of mass action to the ionization equilibrium for the weak acid in water: + - HA + H2O H3O + A (4.2) we may write (in dilute solutions [H2O] is essentially constant) [H O+ ][A − ] 3 = K (4.3) [HA] a where Ka is the acid ionization constant (constant at any given temperature). This expression is valid for + - all aqueous solutions containing hydronium ions (H3O ), A ions, and the un-ionized molecules HA. -

UNIT 11 CHEMISTRY of D- Andf-BLOCK ELEMENTS
UNIT 11 CHEMISTRY OF d- ANDf-BLOCK ELEMENTS Structure 11.1 Introduction Objectives 11.2 Transition and Inner Transition Elements - An Introduction 11.3 IUPAC Nomenclature of 6d Transition Series Elements 11.4 .Electronic Configuration of d-Block and f-Block Elements Electronic Configurations of Transition Elements and Ions Electronic Configurations of Lanthanide and Actinide Elements 11.5 Periodic Trends in Properties Atomic Radii and Ioaic Rad~i Melting and Boiling Points Enthalpies of Ionization Oxidation States Colour of the Complexes Magnetic Properties Catalytic Properties Formation of Complexes Formation of Interstitial Compounds (Interstitial Solid Solutions) and Alloys (Substitutional Solid Solutions) 11.6 Summary 11.7 Terminal Questions 11.1 INTRODUCTION In last unit we have studies about the periodicity and representative elements. In this unit we will study the chemistry of d and f block elements. First we will study the IUPAC nomenclature of these elements then we will discuss the electronic configuration, periodicity, variation of size, melting and boiling points. We shall also study the ionization energy, electronegativity, electrode potential, oxidation sate of these elements in detail. Objectives After studying this unit, you should be able to: explain the IUPAC nomenclature of d and f block elements, describe the electronic configuration of d and f block elements, outline the general properties of these elements, and discuss the colour, magnetic complex formation catalytic properties. 1 1.2 TRANSITION AND INNER TRANSITION ELEMENTS - AN INTRODUCTION We already know that in the periodic table the elements are classified into four blocks; namely, s-block, p-block, d-block andfiblock, based on the name of atomic orbital that accepts the valence or differentiating electrons. -

22. Atomic Physics and Quantum Mechanics
22. Atomic Physics and Quantum Mechanics S. Teitel April 14, 2009 Most of what you have been learning this semester and last has been 19th century physics. Indeed, Maxwell's theory of electromagnetism, combined with Newton's laws of mechanics, seemed at that time to express virtually a complete \solution" to all known physical problems. However, already by the 1890's physicists were grappling with certain experimental observations that did not seem to fit these existing theories. To explain these puzzling phenomena, the early part of the 20th century witnessed revolutionary new ideas that changed forever our notions about the physical nature of matter and the theories needed to explain how matter behaves. In this lecture we hope to give the briefest introduction to some of these ideas. 1 Photons You have already heard in lecture 20 about the photoelectric effect, in which light shining on the surface of a metal results in the emission of electrons from that surface. To explain the observed energies of these emitted elec- trons, Einstein proposed in 1905 that the light (which Maxwell beautifully explained as an electromagnetic wave) should be viewed as made up of dis- crete particles. These particles were called photons. The energy of a photon corresponding to a light wave of frequency f is given by, E = hf ; where h = 6:626×10−34 J · s is a new fundamental constant of nature known as Planck's constant. The total energy of a light wave, consisting of some particular number n of photons, should therefore be quantized in integer multiples of the energy of the individual photon, Etotal = nhf. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table.