Moles and Molarity 6 Equivalent Weights and Normality 7 Dilution Calculations 8 Standard Solutions 12
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamics of Ion Exchange
Chapter 1 Thermodynamics of Ion Exchange Ayben Kilislioğlu Additional information is available at the end of the chapter http://dx.doi.org/10.5772/53558 1. Introduction 1.1. Ion exchange equilibria During an ion exchange process, ions are essentially stepped from the solvent phase to the solid surface. As the binding of an ion takes place at the solid surface, the rotational and translational freedom of the solute are reduced. Therefore, the entropy change (ΔS) during ion exchange is negative. For ion exchange to be convenient, Gibbs free energy change (ΔG) must be negative, which in turn requires the enthalpy change to be negative because ΔG = ΔH - TΔS. Both enthalpic (ΔHo) and entropic (ΔSo) changes help decide the overall selectivity of the ion-exchange process [Marcus Y., SenGupta A. K. 2004]. Thermodynamics have great efficiency on the impulsion of ion exchange. It also sets the equilibrium distribution of ions between the solution and the solid. A discussion about the role of thermodynamics relevant to both of these phenomena was done by researchers [Araujo R., 2004]. As the basic rule of ion exchange, one type of a free mobile ion of a solution become fixed on the solid surface by releasing a different kind of an ion from the solid surface. It is a reversible process which means that there is no permanent change on the solid surface by the process. Ion exchange has many applications in different fields like enviromental, medical, technological,.. etc. To evaluate the properties and efficiency of the ion exchange one must determine the equilibrium conditions. -

Polymer Exemption Guidance Manual POLYMER EXEMPTION GUIDANCE MANUAL
United States Office of Pollution EPA 744-B-97-001 Environmental Protection Prevention and Toxics June 1997 Agency (7406) Polymer Exemption Guidance Manual POLYMER EXEMPTION GUIDANCE MANUAL 5/22/97 A technical manual to accompany, but not supersede the "Premanufacture Notification Exemptions; Revisions of Exemptions for Polymers; Final Rule" found at 40 CFR Part 723, (60) FR 16316-16336, published Wednesday, March 29, 1995 Environmental Protection Agency Office of Pollution Prevention and Toxics 401 M St., SW., Washington, DC 20460-0001 Copies of this document are available through the TSCA Assistance Information Service at (202) 554-1404 or by faxing requests to (202) 554-5603. TABLE OF CONTENTS LIST OF EQUATIONS............................ ii LIST OF FIGURES............................. ii LIST OF TABLES ............................. ii 1. INTRODUCTION ............................ 1 2. HISTORY............................... 2 3. DEFINITIONS............................. 3 4. ELIGIBILITY REQUIREMENTS ...................... 4 4.1. MEETING THE DEFINITION OF A POLYMER AT 40 CFR §723.250(b)... 5 4.2. SUBSTANCES EXCLUDED FROM THE EXEMPTION AT 40 CFR §723.250(d) . 7 4.2.1. EXCLUSIONS FOR CATIONIC AND POTENTIALLY CATIONIC POLYMERS ....................... 8 4.2.1.1. CATIONIC POLYMERS NOT EXCLUDED FROM EXEMPTION 8 4.2.2. EXCLUSIONS FOR ELEMENTAL CRITERIA........... 9 4.2.3. EXCLUSIONS FOR DEGRADABLE OR UNSTABLE POLYMERS .... 9 4.2.4. EXCLUSIONS BY REACTANTS................ 9 4.2.5. EXCLUSIONS FOR WATER-ABSORBING POLYMERS........ 10 4.3. CATEGORIES WHICH ARE NO LONGER EXCLUDED FROM EXEMPTION .... 10 4.4. MEETING EXEMPTION CRITERIA AT 40 CFR §723.250(e) ....... 10 4.4.1. THE (e)(1) EXEMPTION CRITERIA............. 10 4.4.1.1. LOW-CONCERN FUNCTIONAL GROUPS AND THE (e)(1) EXEMPTION................. -

Chapter 20 Electrochemistry
Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method of half-reactions. Sketch a voltaic cell and identify its cathode, anode, and the directions in which electrons and ions move. o Calculate standard emfs (cell potentials), E cell, from standard reduction potentials. Use reduction potentials to predict whether a redox reaction is spontaneous. o o Relate E cell to DG and equilibrium constants. Calculate emf under nonstandard conditions. Identify the components of common batteries. Describe the construction of a lithium-ion battery and explain how it works. Describe the construction of a fuel cell and explain how it generates electrical energy. Explain how corrosion occurs and how it is prevented by cathodic protection. Describe the reactions in electrolytic cells. Relate the amounts of products and reactants in redox reactions to electrical charge. Electrochemistry Electrochemistry is the study of the relationships between electricity and chemical reactions. • It includes the study of both spontaneous and nonspontaneous processes. 1 Redox reactions: assigning oxidation numbers Oxidation numbers help keep track of what species loses electrons and what species gains them. • An element is oxidized when the oxidation number increases • An element is reduced when the oxidation number decreases • an oxidizing agent causes another element to be oxidized • a reducing agent causes another element to be reduced. Assigning oxidation numbers (sect. 4.4) 1. Elemental form, each atom has ox. # = 0. Zn O2 O3 I2 S8 P4 2. Simple ions, = charge on ion. -1 for Cl-, +2 for Mg2+ 3. -

Energy and the Hydrogen Economy
Energy and the Hydrogen Economy Ulf Bossel Fuel Cell Consultant Morgenacherstrasse 2F CH-5452 Oberrohrdorf / Switzerland +41-56-496-7292 and Baldur Eliasson ABB Switzerland Ltd. Corporate Research CH-5405 Baden-Dättwil / Switzerland Abstract Between production and use any commercial product is subject to the following processes: packaging, transportation, storage and transfer. The same is true for hydrogen in a “Hydrogen Economy”. Hydrogen has to be packaged by compression or liquefaction, it has to be transported by surface vehicles or pipelines, it has to be stored and transferred. Generated by electrolysis or chemistry, the fuel gas has to go through theses market procedures before it can be used by the customer, even if it is produced locally at filling stations. As there are no environmental or energetic advantages in producing hydrogen from natural gas or other hydrocarbons, we do not consider this option, although hydrogen can be chemically synthesized at relative low cost. In the past, hydrogen production and hydrogen use have been addressed by many, assuming that hydrogen gas is just another gaseous energy carrier and that it can be handled much like natural gas in today’s energy economy. With this study we present an analysis of the energy required to operate a pure hydrogen economy. High-grade electricity from renewable or nuclear sources is needed not only to generate hydrogen, but also for all other essential steps of a hydrogen economy. But because of the molecular structure of hydrogen, a hydrogen infrastructure is much more energy-intensive than a natural gas economy. In this study, the energy consumed by each stage is related to the energy content (higher heating value HHV) of the delivered hydrogen itself. -

Introduction to Chemistry
Introduction to Chemistry Author: Tracy Poulsen Digital Proofer Supported by CK-12 Foundation CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook Introduction to Chem... materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based Authored by Tracy Poulsen collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and 8.5" x 11.0" (21.59 x 27.94 cm) distribution of high-quality educational content that will serve both as core text as well as provide Black & White on White paper an adaptive environment for learning. 250 pages ISBN-13: 9781478298601 Copyright © 2010, CK-12 Foundation, www.ck12.org ISBN-10: 147829860X Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made Please carefully review your Digital Proof download for formatting, available to Users in accordance with the Creative Commons Attribution/Non-Commercial/Share grammar, and design issues that may need to be corrected. Alike 3.0 Unported (CC-by-NC-SA) License (http://creativecommons.org/licenses/by-nc- sa/3.0/), as amended and updated by Creative Commons from time to time (the “CC License”), We recommend that you review your book three times, with each time focusing on a different aspect. which is incorporated herein by this reference. Specific details can be found at http://about.ck12.org/terms. Check the format, including headers, footers, page 1 numbers, spacing, table of contents, and index. 2 Review any images or graphics and captions if applicable. -
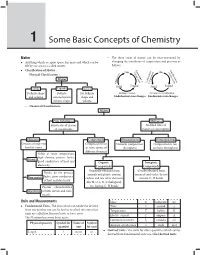
1 Some Basic Concepts of Chemistry
Some Basic Concepts of Chemistry 1 1 Some Basic Concepts of Chemistry Matter – The three states of matter can be inter-converted by Anything which occupies space, has mass and which can be changing the conditions of temperature and pressure as felt by our senses is called matter. follows : Ev Liq Classification of Matter : Co GasGVa ap as uefacti n po orat tion ndensa – tion Physical Classification : io riza at io on n o tion tion Matter lim ndensa or r Deposi Co Sub or Solid Liquid Solid Liquid Solids Liquids Gases Definite shape Definite No definite Melting or Fusion Freezing or Crystallization and volume volume but no shape and Endothermic state changes Exothermic state changes definite shape volume – Chemical Classification : Matter Pure Substances Mixtures Fixed ratio of masses No fixed ratio of of constituents masses of constituents Elements Compounds Homogeneous Heterogeneous Consists of only one Composed of two Uniform composition Composition is not kind of atoms or more atoms of throughout uniform throughout different elements Solids at room temperature, high density, possess lustre, Metals good conductors of heat and Organic Inorganic electricity Compounds Compounds Originally obtained from Usually obtained from Brittle, do not possess animals and plants, contain minerals and rocks, do not lustre, poor conductors Non-metals carbon and few other elements contain C–H bonds of heat and electricity like H, O, S, N, X (halogens), Possess characteristics etc. having C–H bonds Metalloids of both metals and non- metals Units and Measurements Mass m kilogram kg Fundamental Units : The units which can neither be derived Time t second s from one another nor can be further resolved into any other Temperature T kelvin K units are called fundamental units or basic units. -

Basic Concepts and Laws of Chemistry. Guidelines and Objectives for Self-Study Courses for Students in All Specialties / O.Y
THE MINISTRY OF EDUCATION AND SCIENCE OF UKRAINE STATE HIGHER EDUCATIONAL INSTITUTION «NATIONAL MINING UNIVERSITY» O.Y. Svietkina, O.B Netyaga, G.V Tarasova BASIC CONCEPTS AND LAWS OF CHEMISTRY. Guidelines and objectives for self-study courses for students in all specialties Dnipro 2016 THE MINISTRY OF EDUCATION AND SCIENCE OF UKRAINE STATE HIGHER EDUCATIONAL INSTITUTION «NATIONAL MINING UNIVERSITY» FACULTY OF GEOLOGICAL PROSPECTING Departament of Chemestry O. Y. Svietkina, O.B Netyaga, G.V Tarasova BASIC CONCEPTS AND LAWS OF CHEMISTRY. Guidelines and objectives for self-study courses for students in all specialties Dnipro NMU 2016 Svietkina O. Y. Basic concepts and laws of chemistry. Guidelines and objectives for self-study courses for students in all specialties / O.Y. Svietkina, O.B. Netyaga, G.V. Tarasova; Ministry of eduk. and sien of Ukrain, Nation. min. univer. – D . : NMU, 2016. – 20 p. Автори: О.Ю. Свєткіна, проф., д-р техн. наук (передмова, розділ 2); О.Б. Нетяга, старш. викл. (розділ 1); Г.В. Тарасова, асист. (розділ 2). Затверджено методичною комісією з галузі знань 10. Природничі науки за поданням кафедри хімії (протокол № 3 від 08.11.2016). The theoretical themes of "Basic concepts and laws of chemistry", are examples of solving common tasks, in order to consolidate the material there are presented self-study tasks for solving. Розглянуто теоретичні положення теми «Основні поняття й закони хімії», наведено приклади розв’язку типових задач з метою закріплення матеріалу, подано задачі для самостійного розв’язування. Відповідальна за випуск завідувач кафедри хімії, д-р техн. наук, проф. О.Ю. Свєткіна. Introduction Chemistry studies such form of substance motion which assumes qualitative change of matters i.e. -

(I) Determination of the Equivalent Weight and Pka of an Organic Acid
Experiment 4 (i) Determination of the Equivalent Weight and pKa of an Organic Acid Discussion This experiment is an example of a common research procedure. Chemists often use two or more analytical techniques to study the same system. These experiments can give complementary qualitative and quantitative information concerning an unknown substance. I. Titration of Acids and Bases in Aqueous Solutions The almost instantaneous reaction between acids and bases in aqueous solution produce changes in pH which one can monitor. Two techniques are useful for detecting the equivalence point: (1) colorimetry, using an acid-base color indicator - a dye which undergoes a sharp change in color in a region of pH covering the equivalence point and (2) potentiometry, using a potentiometer (pH meter) to record the sharp change at the equivalence point in the potential difference between an electrode (usually a glass electrode) and the solution whose pH is undergoing change as a result of the addition of acid or base. For example, in the case of the titration of a weak monoprotic acid HA using sodium hydroxide solution we may write: + - NaOH + HA → Na + A + H2O (4.1) Applying the law of mass action to the ionization equilibrium for the weak acid in water: + - HA + H2O H3O + A (4.2) we may write (in dilute solutions [H2O] is essentially constant) [H O+ ][A − ] 3 = K (4.3) [HA] a where Ka is the acid ionization constant (constant at any given temperature). This expression is valid for + - all aqueous solutions containing hydronium ions (H3O ), A ions, and the un-ionized molecules HA. -

Battle of the Acids Weak Acids and Bases SCIENTIFIC
Battle of the Acids Weak Acids and Bases SCIENTIFIC Introduction Not all acids are created equal. This demonstration compares the “frothing and foaming” activity of different acids with calcium carbonate and examines their behavior in the presence of their conjugate bases to distinguish strong versus weak acids. The use of a “rainbow acid” universal indicator produces a rainbow spectrum of color changes as the mixtures react. Concepts • Strong acid • Weak acid • Conjugate base • pH Materials (for each demonstration) Acetic acid, CH3COOH, 1 M, 400 mL Water, distilled or deionized Calcium carbonate, CaCO3, 40 g Demonstration tray, large Hydrochloric acid, HCl, 1 M, 400 mL Graduated cylinder, 250-mL “Rainbow acid” universal indicator, 5 mL Hydrometer cylinders, 600-mL, 4 (includes accompanying color chart) Pipet, Beral-type Sodium acetate, NaCH3CO2, 16 g Weighing dishes or small beakers, 6 Sodium chloride, NaCl, 12 g Safety Precautions Hydrochloric acid and acetic acid solutions are toxic and corrosive. Avoid contact with skin and eyes. “Rainbow-acid” universal indicator solution is an alcohol-based solution and is flammable. Avoid contact with flames or other ignition sources. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. Please review current Material Safety Data Sheets for additional safety, handling, and disposal information. Preparation To save time in the presentation, pre-measure the amounts of solids needed for the demonstration. Weigh out 12 g of sodium chloride, 16 g of sodium acetate, and 4 × 10-g samples of calcium carbonate in separate, labeled weighing dishes or small beakers. Procedure 1. Obtain 4 large hydrometer cylinders or tall-form beakers and place them on a large demonstration tray. -

PHARMACY CALCULATIONS •Explain Importance of Calculations in Determining Days Supply •Describe Consequences of Calculation Errors
7/7/2016 THE OBJECTIVES INCLUDE THE FOLLOWING: •Discuss importance of calculations in a pharmacy practice. •Outline steps to avoid calculation errors 2016 ANNUAL MEETING •Explain importance of reading the prescription carefully then performing calculations PHARMACY CALCULATIONS •Explain importance of calculations in determining days supply •Describe consequences of calculation errors. •Perform calculations during the presentation 2016 ANNUAL MEETING 4 ADDITIONAL LEARNING OBJECTIVES •Understand how the concentration of electrolyte 2016 ANNUAL MEETING solutions are expressed and calculations of m.Eq. •Understand the importance of iso-tonicity in SUNIL S. JAMBHEKAR, B. PHARM.; M.S., PH.D ophthalmic solutions. PROFESSOR, PHARMACEUTICAL SCIENCES LECOM BRADENTON, SCHOOL OF PHARMACY •Perform calculations, using a prescription, to BRADENTON, FL34202 determine the quantity of a liquid product required [email protected] to fill the prescription accurately. 2016 ANNUAL MEETING 5 ADDITIONAL LEARNING OBJECTIVES •Perform calculations, using a prescription, to 2016 ANNUAL MEETING determine the # of day supply for a specific DISCLOSURE product. I DO NOT HAVE A VESTED INTEREST IN OR AFFILIATION •Perform calculations to determine m.Eq WITH ANY CORPORATE ORGANIZATION OFFERING FINANCIAL SUPPORT OR GRANT MONIES FOR THIS CONTINUING strength of an electrolyte solution. EDUCATION ACTIVITY, OR ANY AFFILIATION WITH AN ORGANIZATION WHOSE PHILOSOPHY COULD POTENTIALLY •Perform calculations to prepare an isotonic BIAS MY PRESENTATION ophthalmic solution. 2016 ANNUAL MEETING 6 1 7/7/2016 IMPORTANCE OF CALCULATIONS IN THE PHARMACY PRACTICE: •It is consistent with the practice and philosophy of pharmaceutical care. •Insurance companies will reimburse you or •Patient safety is of utmost importance. your employer only for the quantity written on the prescription and not for the quantity •Your pharmacy is the last check point for the dispensed. -

Stoichiometry of Chemical Reactions 175
Chapter 4 Stoichiometry of Chemical Reactions 175 Chapter 4 Stoichiometry of Chemical Reactions Figure 4.1 Many modern rocket fuels are solid mixtures of substances combined in carefully measured amounts and ignited to yield a thrust-generating chemical reaction. (credit: modification of work by NASA) Chapter Outline 4.1 Writing and Balancing Chemical Equations 4.2 Classifying Chemical Reactions 4.3 Reaction Stoichiometry 4.4 Reaction Yields 4.5 Quantitative Chemical Analysis Introduction Solid-fuel rockets are a central feature in the world’s space exploration programs, including the new Space Launch System being developed by the National Aeronautics and Space Administration (NASA) to replace the retired Space Shuttle fleet (Figure 4.1). The engines of these rockets rely on carefully prepared solid mixtures of chemicals combined in precisely measured amounts. Igniting the mixture initiates a vigorous chemical reaction that rapidly generates large amounts of gaseous products. These gases are ejected from the rocket engine through its nozzle, providing the thrust needed to propel heavy payloads into space. Both the nature of this chemical reaction and the relationships between the amounts of the substances being consumed and produced by the reaction are critically important considerations that determine the success of the technology. This chapter will describe how to symbolize chemical reactions using chemical equations, how to classify some common chemical reactions by identifying patterns of reactivity, and how to determine the quantitative relations between the amounts of substances involved in chemical reactions—that is, the reaction stoichiometry. 176 Chapter 4 Stoichiometry of Chemical Reactions 4.1 Writing and Balancing Chemical Equations By the end of this section, you will be able to: • Derive chemical equations from narrative descriptions of chemical reactions. -

WO 2019/055177 Al 21 March 2019 (21.03.2019) W 1P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2019/055177 Al 21 March 2019 (21.03.2019) W 1P O PCT (51) International Patent Classification: Declarations under Rule 4.17: C08G 65/02 (2006.01) C08G 18/32 (2006.01) — as to applicant's entitlement to apply for and be granted a C08G 65/26 (2006.01) patent (Rule 4.17(H)) (21) International Application Number: — as to the applicant's entitlement to claim the priority of the PCT/US20 18/047252 earlier application (Rule 4.17(Hi)) (22) International Filing Date: Published: 2 1 August 2018 (21.08.2018) — with international search report (Art. 21(3)) (25) Filing Language: English (26) Publication Language: English (30) Priority Data: 62/559,109 15 September 2017 (15.09.2017) US (71) Applicant: DOW GLOBAL TECHNOLOGIES LLC [US/US]; 2040 Dow Center, Midland, Michigan 48674 (US). (72) Inventors: MASY, Jean-Paul; Herbert H . Dowweg 5, 4542 NM Hoek, Postbus 48, 4530AA Terneuzen (NL). BABB, David A.; 230 1N . Brazosport Boulevard, Freeport, Texas 77541 (US). (74) Agent: Gary C Cohn PLLC; 325 7th Avenue, #203, San Diego, California 92101 (US). (81) Designated States (unless otherwise indicated, for every kind of national protection available) : AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW.