Chromosome Evolution at the Origin of the Ancestral Vertebrate Genome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Phylum Vertebrata: a Case for Zoological Recognition Naoki Irie1,2* , Noriyuki Satoh3 and Shigeru Kuratani4
Irie et al. Zoological Letters (2018) 4:32 https://doi.org/10.1186/s40851-018-0114-y REVIEW Open Access The phylum Vertebrata: a case for zoological recognition Naoki Irie1,2* , Noriyuki Satoh3 and Shigeru Kuratani4 Abstract The group Vertebrata is currently placed as a subphylum in the phylum Chordata, together with two other subphyla, Cephalochordata (lancelets) and Urochordata (ascidians). The past three decades, have seen extraordinary advances in zoological taxonomy and the time is now ripe for reassessing whether the subphylum position is truly appropriate for vertebrates, particularly in light of recent advances in molecular phylogeny, comparative genomics, and evolutionary developmental biology. Four lines of current research are discussed here. First, molecular phylogeny has demonstrated that Deuterostomia comprises Ambulacraria (Echinodermata and Hemichordata) and Chordata (Cephalochordata, Urochordata, and Vertebrata), each clade being recognized as a mutually comparable phylum. Second, comparative genomic studies show that vertebrates alone have experienced two rounds of whole-genome duplication, which makes the composition of their gene family unique. Third, comparative gene-expression profiling of vertebrate embryos favors an hourglass pattern of development, the most conserved stage of which is recognized as a phylotypic period characterized by the establishment of a body plan definitively associated with a phylum. This mid-embryonic conservation is supported robustly in vertebrates, but only weakly in chordates. Fourth, certain complex patterns of body plan formation (especially of the head, pharynx, and somites) are recognized throughout the vertebrates, but not in any other animal groups. For these reasons, we suggest that it is more appropriate to recognize vertebrates as an independent phylum, not as a subphylum of the phylum Chordata. -

Brains of Primitive Chordates 439
Brains of Primitive Chordates 439 Brains of Primitive Chordates J C Glover, University of Oslo, Oslo, Norway although providing a more direct link to the evolu- B Fritzsch, Creighton University, Omaha, NE, USA tionary clock, is nevertheless hampered by differing ã 2009 Elsevier Ltd. All rights reserved. rates of evolution, both among species and among genes, and a still largely deficient fossil record. Until recently, it was widely accepted, both on morpholog- ical and molecular grounds, that cephalochordates Introduction and craniates were sister taxons, with urochordates Craniates (which include the sister taxa vertebrata being more distant craniate relatives and with hemi- and hyperotreti, or hagfishes) represent the most chordates being more closely related to echinoderms complex organisms in the chordate phylum, particu- (Figure 1(a)). The molecular data only weakly sup- larly with respect to the organization and function of ported a coherent chordate taxon, however, indicat- the central nervous system. How brain complexity ing that apparent morphological similarities among has arisen during evolution is one of the most chordates are imposed on deep divisions among the fascinating questions facing modern science, and it extant deuterostome taxa. Recent analysis of a sub- speaks directly to the more philosophical question stantially larger number of genes has reversed the of what makes us human. Considerable interest has positions of cephalochordates and urochordates, pro- therefore been directed toward understanding the moting the latter to the most closely related craniate genetic and developmental underpinnings of nervous relatives (Figure 1(b)). system organization in our more ‘primitive’ chordate relatives, in the search for the origins of the vertebrate Comparative Appearance of Brains, brain in a common chordate ancestor. -
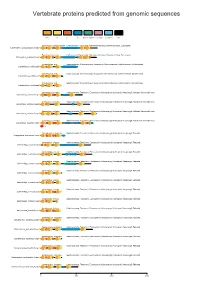
Vertebrate Proteins Predicted from Genomic Sequences
Vertebrate proteins predicted from genomic sequences VWD C8 TIL PTS Mucin2_WxxW F5_F8_type_C FCGBP_N VWC Lethenteron_camtschaticum Cyclostomata; Hyperoartia; Petromyzontiformes; Petromyzontidae; Lethenteron Lethenteron_camtschaticum.0.pep1 Petromyzon_marinus Cyclostomata; Hyperoartia; Petromyzontiformes; Petromyzontidae; Petromyzon Petromyzon_marinus.0.pep1 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep1 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep2 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep3 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep1 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep2 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep3 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.1.pep1 TILa Cynoglossus_semilaevis Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Teleostei; Cynoglossus_semilaevis.1.pep1 -

Copyrighted Material
Index INDEX Note: page numbers in italics refer to fi gures, those in bold refer to tables and boxes. abducens nerve 55 activity cycles 499–522 inhibition 485 absorption effi ciency 72 annual patterns 515, 516, 517–22 interactions 485–6 abyssal zone 393 circadian rhythms 505 prey 445 Acanthaster planci (Crown-of-Thorns Starfi sh) diel patterns 499, 500, 501–2, 503–4, reduction 484 579 504–7 aggressive mimicry 428, 432–3 Acanthocybium (Wahoo) 15 light-induced 499, 500, 501–2, 503–4, aggressive resemblance 425–6 Acanthodii 178, 179 505 aglomerular 52 Acanthomorpha 284–8, 289 lunar patterns 507–9 agnathans Acanthopterygii 291–325 seasonal 509–15 gills 59, 60 Atherinomorpha 293–6 semilunar patterns 507–9 osmoregulation 101, 102 characteristics 291–2 supra-annual patterns 515, 516, 517–22 phylogeny 202 distribution 349, 350 tidal patterns 506–7 ventilation 59, 60 jaws 291 see also migration see also hagfi shes; lampreys Mugilomorpha 292–3, 294 adaptive response 106 agnathous fi shes see jawless fi shes pelagic 405 adaptive zones 534 agonistic interactions 83–4, 485–8 Percomorpha 296–325 adenohypophysis 91, 92 chemically mediated 484 pharyngeal jaws 291 adenosine triphosphate (ATP) 57 sound production 461–2 phylogeny 292, 293, 294 adipose fi n 35 visual 479 spines 449, 450 adrenocorticotropic hormone (ACTH) 92 agricultural chemicals 605 Acanthothoraciformes 177 adrianichthyids 295 air breathing 60, 61–2, 62–4 acanthurids 318–19 adult fi shes 153, 154, 155–7 ammonia production 64, 100–1 Acanthuroidei 12, 318–19 death 156–7 amphibious 60 Acanthurus bahianus -

Constraints on the Timescale of Animal Evolutionary History
Palaeontologia Electronica palaeo-electronica.org Constraints on the timescale of animal evolutionary history Michael J. Benton, Philip C.J. Donoghue, Robert J. Asher, Matt Friedman, Thomas J. Near, and Jakob Vinther ABSTRACT Dating the tree of life is a core endeavor in evolutionary biology. Rates of evolution are fundamental to nearly every evolutionary model and process. Rates need dates. There is much debate on the most appropriate and reasonable ways in which to date the tree of life, and recent work has highlighted some confusions and complexities that can be avoided. Whether phylogenetic trees are dated after they have been estab- lished, or as part of the process of tree finding, practitioners need to know which cali- brations to use. We emphasize the importance of identifying crown (not stem) fossils, levels of confidence in their attribution to the crown, current chronostratigraphic preci- sion, the primacy of the host geological formation and asymmetric confidence intervals. Here we present calibrations for 88 key nodes across the phylogeny of animals, rang- ing from the root of Metazoa to the last common ancestor of Homo sapiens. Close attention to detail is constantly required: for example, the classic bird-mammal date (base of crown Amniota) has often been given as 310-315 Ma; the 2014 international time scale indicates a minimum age of 318 Ma. Michael J. Benton. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Philip C.J. Donoghue. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Robert J. -

Epithelial Sodium Channel (Enac) Family: Phylogeny, Structure–Function, Tissue Distribution, and Associated Inherited Diseases
Gene 579 (2016) 95–132 Contents lists available at ScienceDirect Gene journal homepage: www.elsevier.com/locate/gene Gene wiki review Epithelial sodium channel (ENaC) family: Phylogeny, structure–function, tissue distribution, and associated inherited diseases Israel Hanukoglu a,⁎, Aaron Hanukoglu b,c a Laboratory of Cell Biology, Faculty of Natural Sciences, Ariel University, Ariel, Israel b Division of Pediatric Endocrinology, E. Wolfson Medical Center, Holon, Israel c Sackler School of Medicine, Tel-Aviv University, Tel Aviv, Israel article info abstract Article history: The epithelial sodium channel (ENaC) is composed of three homologous subunits and allows the flow of Na+ ions Received 7 September 2015 across high resistance epithelia, maintaining body salt and water homeostasis. ENaC dependent reabsorption of Received in revised form 20 December 2015 Na+ in the kidney tubules regulates extracellular fluid (ECF) volume and blood pressure by modulating osmolar- Accepted 22 December 2015 ity. In multi-ciliated cells, ENaC is located in cilia and plays an essential role in the regulation of epithelial surface Available online 7 January 2016 liquid volume necessary for cilial transport of mucus and gametes in the respiratory and reproductive tracts respectively. Keywords: Ion channels The subunits that form ENaC (named as alpha, beta, gamma and delta, encoded by genes SCNN1A, SCNN1B, Epithelia SCNN1G, and SCNN1D) are members of the ENaC/Degenerin superfamily. The earliest appearance of ENaC Evolution orthologs is in the genomes of the most ancient vertebrate taxon, Cyclostomata (jawless vertebrates) including Transmembrane proteins lampreys, followed by earliest representatives of Gnathostomata (jawed vertebrates) including cartilaginous Kidney sharks. Among Euteleostomi (bony vertebrates), Actinopterygii (ray finned-fishes) branch has lost ENaC genes. -

Tunicate Mitogenomics and Phylogenetics: Peculiarities of the Herdmania Momus Mitochondrial Genome and Support for the New Chordate Phylogeny
Tunicate mitogenomics and phylogenetics: peculiarities of the Herdmania momus mitochondrial genome and support for the new chordate phylogeny. Tiratha Raj Singh, Georgia Tsagkogeorga, Frédéric Delsuc, Samuel Blanquart, Noa Shenkar, Yossi Loya, Emmanuel Douzery, Dorothée Huchon To cite this version: Tiratha Raj Singh, Georgia Tsagkogeorga, Frédéric Delsuc, Samuel Blanquart, Noa Shenkar, et al.. Tu- nicate mitogenomics and phylogenetics: peculiarities of the Herdmania momus mitochondrial genome and support for the new chordate phylogeny.. BMC Genomics, BioMed Central, 2009, 10, pp.534. 10.1186/1471-2164-10-534. halsde-00438100 HAL Id: halsde-00438100 https://hal.archives-ouvertes.fr/halsde-00438100 Submitted on 2 Dec 2009 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. BMC Genomics BioMed Central Research article Open Access Tunicate mitogenomics and phylogenetics: peculiarities of the Herdmania momus mitochondrial genome and support for the new chordate phylogeny Tiratha Raj Singh†1, Georgia Tsagkogeorga†2, Frédéric Delsuc2, Samuel Blanquart3, Noa -

Bos Taurus, Cattle, Cow at Geochembio: Taxonomy, Brief Facts
http://www.GeoChemBio.com: Bos taurus, cattle, cow ● Taxonomy ● Brief facts ● Developmental stages ● Digestive system ● Diagram of digestive system ● Some Bovidae species ● Bovidae species ● Cattle domestication ● Cattle domestication diagram ● Photo gallery Taxonomy cellular organisms - Eukaryota - Fungi/Metazoa group - Metazoa - Eumetazoa - Bilateria - Coelomata - Deuterostomia - Chordata - Craniata - Vertebrata - Gnathostomata - Teleostomi - Euteleostomi - Sarcopterygii - Tetrapoda - Amniota - Mammalia - Theria - Eutheria - Laurasiatheria - Cetartiodactyla - Ruminantia - Pecora - Bovidae - Bovinae - Bos - Bos taurus The two principal taxonomic groups of domestic cattle are Bos taurus (taurine cattle) and Bos indicus (zebu cattle). General description ● Domestic cows are common throughout the world. They are grown and bred for food - meat and milk production, as well as for working - plowing and moving heavy loads. ● Domestic cows are social animals and live in herds which are structured according to a dominance hierarchy. ● Cows feed on grasses and other herbaceous plants. An average cow can consume about 70 kg of grass in an 8 hour day. Cows are ruminants. They have a four chambered stomach. Cow's milk allergy vs. cow's milk intolerance ● Cow's milk allergy and cow's milk intolerance are two different concepts that are used interchangeably. The former is an immunologically mediated reaction to the milk's contents, not unlike to any other allergic reactions, for example, to eggs, corn, nuts, etc. The latter is non-immunologic reaction to the cow's milk, the most common cause of which is deficiency of lactase - the enzyme that breaks down one of the main nutrients of the milk - lactose. Importance of bovine genome ● The bovine genome serves as a reference non-primate, non-rodent, eutherian genome. -

The Whale Shark Genome Reveals Patterns of Vertebrate Gene Family Evolution
bioRxiv preprint doi: https://doi.org/10.1101/685743; this version posted June 28, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. The whale shark genome reveals patterns of vertebrate gene family evolution 1 2 3 4 4,5 Milton Tan *, Anthony K. Redmond , Helen Dooley , Ryo Nozu , Keiichi Sato , Shigehiro 6 7 7 8 9 Kuraku , Sergey Koren , Adam M. Phillippy , Alistair D.M. Dove , Timothy D. Read 1. Illinois Natural History Survey at University of Illinois Urbana-Champaign, Champaign, IL, USA. 2. Smurfit Institute of Genetics, Trinity College Dublin, Dublin, Ireland. 3. University of Maryland School of Medicine, Institute of Marine & Environmental Technology, Baltimore, MD, USA. 4. Okinawa Churashima Research Center, Okinawa Churashima Foundation, Okinawa, Japan. 5. Okinawa Churaumi Aquarium, Motobu, Okinawa, Japan 6. RIKEN Center for Biosystems Dynamics Research (BDR), RIKEN, Kobe, Japan. 7. National Human Genome Research Institute, Bethesda, MD, USA. 8. Georgia Aquarium, Atlanta, GA, USA. 9. Department of Infectious Diseases, Emory University School of Medicine, Atlanta, GA, USA. Keywords: fish, Elasmobranchii, Chondrichthyes, Cartilaginous Fishes, Gnathostomata, comparative genomics, innate immunity, gigantism Abstract Due to their key phylogenetic position, cartilaginous fishes, which includes the largest fish species Rhincodon typus (whale shark), are an important vertebrate lineage for understanding the origin and evolution of vertebrates. However, until recently, this lineage has been understudied in vertebrate genomics. Using newly-generated long read sequences, we produced the best gapless cartilaginous fish genome assembly to date. -

Punctuated Emergences of Genetic and Phenotypic Innovations in Eumetazoan, Bilaterian, Euteleostome, and Hominidae Ancestors
GBE Punctuated Emergences of Genetic and Phenotypic Innovations in Eumetazoan, Bilaterian, Euteleostome, and Hominidae Ancestors Yvan Wenger and Brigitte Galliot* Department of Genetics and Evolution, Institute of Genetics and Genomics in Geneva (iGE3), University of Geneva, Geneva, Switzerland *Corresponding author: E-mail: [email protected]. Accepted: September 12, 2013 Data deposition: Sequences of the 6,071 Hydra-human orthologs were deposited at European Nucleotide Archive (ENA), sequencing project PRJEB446, individual accession numbers HAAD01000001–HAAD01006071. Sequences of the 45,269 Hydra RNA-seq transcripts were also de- posited at European Nucleotide Archive (ENA) sequencing project PRJEB445, individual accession numbers HAAC01000001–HAAC01045269. Abstract Phenotypic traits derive from the selective recruitment of genetic materials over macroevolutionary times, and protein-coding genes constitute an essential component of these materials. We took advantage of the recent production of genomic scale data from sponges and cnidarians, sister groups from eumetazoans and bilaterians, respectively, to date the emergence of human proteins and to infer the timing of acquisition of novel traits through metazoan evolution. Comparing the proteomes of 23 eukaryotes, we find that 33% human proteins have an ortholog in nonmetazoan species. This premetazoan proteome associates with 43% of all annotated human biological processes. Subsequently, four major waves of innovations can be inferred in the last common ancestors of eumetazoans, -

The Whale Shark Genome Reveals Patterns of Vertebrate Gene Family
RESEARCH ARTICLE The whale shark genome reveals patterns of vertebrate gene family evolution Milton Tan1*, Anthony K Redmond2, Helen Dooley3, Ryo Nozu4, Keiichi Sato4,5, Shigehiro Kuraku6, Sergey Koren7, Adam M Phillippy7, Alistair DM Dove8, Timothy Read9 1Illinois Natural History Survey at University of Illinois Urbana-Champaign, Champaign, United States; 2Smurfit Institute of Genetics, Trinity College Dublin, Dublin, Ireland; 3University of Maryland School of Medicine, Institute of Marine & Environmental Technology, Baltimore, United States; 4Okinawa Churashima Research Center, Okinawa Churashima Foundation, Okinawa, Japan; 5Okinawa Churaumi Aquarium, Motobu, Okinawa, Japan; 6RIKEN Center for Biosystems Dynamics Research (BDR), RIKEN, Kobe, Japan; 7National Human Genome Research Institute, National Institutes of Health, Bethesda, United States; 8Georgia Aquarium, Atlanta, United States; 9Department of Infectious Diseases, Emory University School of Medicine, Atlanta, United States Abstract Chondrichthyes (cartilaginous fishes) are fundamental for understanding vertebrate evolution, yet their genomes are understudied. We report long-read sequencing of the whale shark genome to generate the best gapless chondrichthyan genome assembly yet with higher contig contiguity than all other cartilaginous fish genomes, and studied vertebrate genomic evolution of ancestral gene families, immunity, and gigantism. We found a major increase in gene families at the origin of gnathostomes (jawed vertebrates) independent of their genome duplication. We studied vertebrate pathogen recognition receptors (PRRs), which are key in initiating innate immune defense, and found diverse patterns of gene family evolution, demonstrating that adaptive *For correspondence: immunity in gnathostomes did not fully displace germline-encoded PRR innovation. We also [email protected] discovered a new toll-like receptor (TLR29) and three NOD1 copies in the whale shark. -

Development, Anatomy, and Phylogenetic Relationships of Jawless Vertebrates and Tests of Hypotheses About Early Vertebrate Evolution
Development, Anatomy, and Phylogenetic Relationships of Jawless Vertebrates and Tests of Hypotheses about Early Vertebrate Evolution by Tetsuto Miyashita A thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Systematics and Evolution Department of Biological Sciences University of Alberta © Tetsuto Miyashita, 2018 ii ABSTRACT The origin and early evolution of vertebrates remain one of the central questions of comparative biology. This clade, which features a breathtaking diversity of complex forms, has generated profound, unresolved questions, including: How are major lineages of vertebrates related to one another? What suite of characters existed in the last common ancestor of all living vertebrates? Does information from seemingly ‘primitive’ groups — jawless vertebrates, cartilaginous fishes, or even invertebrate outgroups — inform us about evolutionary transitions to novel morphologies like the neural crest or jaw? Alfred Romer once likened a search for the elusive vertebrate archetype to a study of the Apocalypse: “That way leads to madness.” I attempt to address these questions using extinct and extant cyclostomes (hagfish, lampreys, and their kin). As the sole living lineage of jawless vertebrates, cyclostomes diverged during the earliest phases of vertebrate evolution. However, precise relationships and evolutionary scenarios remain highly controversial, due to their poor fossil record and specialized morphology. Through a comparative analysis of embryos, I identified significant developmental similarities and differences between hagfish and lampreys, and delineated specific problems to be explored. I attacked the first problem — whether cyclostomes form a clade or represent a grade — in a description and phylogenetic analyses of a new, nearly complete fossil hagfish from the Cenomanian of Lebanon.