Regulation and Evolution of Muscle Development in Tunicates Florian Razy‑Krajka* and Alberto Stolf*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Phylum Vertebrata: a Case for Zoological Recognition Naoki Irie1,2* , Noriyuki Satoh3 and Shigeru Kuratani4
Irie et al. Zoological Letters (2018) 4:32 https://doi.org/10.1186/s40851-018-0114-y REVIEW Open Access The phylum Vertebrata: a case for zoological recognition Naoki Irie1,2* , Noriyuki Satoh3 and Shigeru Kuratani4 Abstract The group Vertebrata is currently placed as a subphylum in the phylum Chordata, together with two other subphyla, Cephalochordata (lancelets) and Urochordata (ascidians). The past three decades, have seen extraordinary advances in zoological taxonomy and the time is now ripe for reassessing whether the subphylum position is truly appropriate for vertebrates, particularly in light of recent advances in molecular phylogeny, comparative genomics, and evolutionary developmental biology. Four lines of current research are discussed here. First, molecular phylogeny has demonstrated that Deuterostomia comprises Ambulacraria (Echinodermata and Hemichordata) and Chordata (Cephalochordata, Urochordata, and Vertebrata), each clade being recognized as a mutually comparable phylum. Second, comparative genomic studies show that vertebrates alone have experienced two rounds of whole-genome duplication, which makes the composition of their gene family unique. Third, comparative gene-expression profiling of vertebrate embryos favors an hourglass pattern of development, the most conserved stage of which is recognized as a phylotypic period characterized by the establishment of a body plan definitively associated with a phylum. This mid-embryonic conservation is supported robustly in vertebrates, but only weakly in chordates. Fourth, certain complex patterns of body plan formation (especially of the head, pharynx, and somites) are recognized throughout the vertebrates, but not in any other animal groups. For these reasons, we suggest that it is more appropriate to recognize vertebrates as an independent phylum, not as a subphylum of the phylum Chordata. -

Colonial Tunicates: Species Guide
SPECIES IN DEPTH Colonial Tunicates Colonial Tunicates Tunicates are small marine filter feeder animals that have an inhalant siphon, which takes in water, and an exhalant siphon that expels water once it has trapped food particles. Tunicates get their name from the tough, nonliving tunic formed from a cellulose-like material of carbohydrates and proteins that surrounds their bodies. Their other name, sea squirts, comes from the fact that many species will shoot LambertGretchen water out of their bodies when disturbed. Massively lobate colony of Didemnum sp. A growing on a rope in Sausalito, in San Francisco Bay. A colony of tunicates is comprised of many tiny sea squirts called zooids. These INVASIVE SEA SQUIRTS individuals are arranged in groups called systems, which form interconnected Star sea squirts (Botryllus schlosseri) are so named because colonies. Systems of these filter feeders the systems arrange themselves in a star. Zooids are shaped share a common area for expelling water like ovals or teardrops and then group together in small instead of having individual excurrent circles of about 20 individuals. This species occurs in a wide siphons. Individuals and systems are all variety of colors: orange, yellow, red, white, purple, grayish encased in a matrix that is often clear and green, or black. The larvae each have eight papillae, or fleshy full of blood vessels. All ascidian tunicates projections that help them attach to a substrate. have a tadpole-like larva that swims for Chain sea squirts (Botryloides violaceus) have elongated, less than a day before attaching itself to circular systems. Each system can have dozens of zooids. -
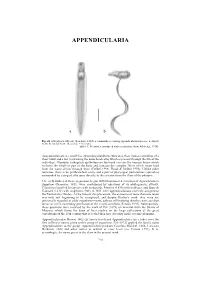
Appendicularia of CTAW
APPENDICULARIA APPENDICULARIA a b Fig. 26. Oikopleura albicans (Leuckart, 1854), a commonly occurring appendicularian species: a, dorsal view; b, lateral view. (Scale bar = 0.1 mm). [after T. Prentiss, reproduced with permission, from Alldredge 1976] Appendicularians are small free swimming planktonic tunicates, their bodies consisting of a short trunk and a tail (containing the notochord cells) which is present through the life of the individual. Glandular (oikoplast) epithelium on the trunk secretes the mucous house which encloses the whole or part of the body and contains the complex filters which strain food from the water driven through them (Deibel 1998; Flood & Deibel 1998). Unlike other tunicates, there is no peribranchial cavity and a pair of pharyngeal perforations (spiracles) surrounded by a ring of cilia open directly to the exterior from the floor of the pharynx. The early studies of these organisms, begun with Chamisso's description of Appendicularia flagellum Chamisso, 1821, were confounded by questions of its phylogenetic affinity. Chamisso classified his species with medusoids, Mertens (1830) with molluscs, and Quoy & Gaimard (1833) with zoophytes. Only in 1851 were appendicularians correctly assigned to the Tunicata by Huxley. At the time of this placement, the existence of more than one taxon was only just beginning to be recognised, and despite Huxley's work, they were not universally regarded as adult organisms—some authors still insisting that they were ascidian larvae or a free swimming generation of the sessile ascidians (Fenaux 1993). Subsequently, these questions were resolved by the work of Fol (1872) on material from the Straits of Messina, which forms the basis of later studies on the large collections of the great expeditions of the 19th century that revealed their true diversity in the oceanic plankton. -

Brains of Primitive Chordates 439
Brains of Primitive Chordates 439 Brains of Primitive Chordates J C Glover, University of Oslo, Oslo, Norway although providing a more direct link to the evolu- B Fritzsch, Creighton University, Omaha, NE, USA tionary clock, is nevertheless hampered by differing ã 2009 Elsevier Ltd. All rights reserved. rates of evolution, both among species and among genes, and a still largely deficient fossil record. Until recently, it was widely accepted, both on morpholog- ical and molecular grounds, that cephalochordates Introduction and craniates were sister taxons, with urochordates Craniates (which include the sister taxa vertebrata being more distant craniate relatives and with hemi- and hyperotreti, or hagfishes) represent the most chordates being more closely related to echinoderms complex organisms in the chordate phylum, particu- (Figure 1(a)). The molecular data only weakly sup- larly with respect to the organization and function of ported a coherent chordate taxon, however, indicat- the central nervous system. How brain complexity ing that apparent morphological similarities among has arisen during evolution is one of the most chordates are imposed on deep divisions among the fascinating questions facing modern science, and it extant deuterostome taxa. Recent analysis of a sub- speaks directly to the more philosophical question stantially larger number of genes has reversed the of what makes us human. Considerable interest has positions of cephalochordates and urochordates, pro- therefore been directed toward understanding the moting the latter to the most closely related craniate genetic and developmental underpinnings of nervous relatives (Figure 1(b)). system organization in our more ‘primitive’ chordate relatives, in the search for the origins of the vertebrate Comparative Appearance of Brains, brain in a common chordate ancestor. -

Sea Squirt Symbionts! Or What I Did on My Summer Vacation… Leah Blasiak 2011 Microbial Diversity Course
Sea Squirt Symbionts! Or what I did on my summer vacation… Leah Blasiak 2011 Microbial Diversity Course Abstract Microbial symbionts of tunicates (sea squirts) have been recognized for their capacity to produce novel bioactive compounds. However, little is known about most tunicate-associated microbial communities, even in the embryology model organism Ciona intestinalis. In this project I explored 3 local tunicate species (Ciona intestinalis, Molgula manhattensis, and Didemnum vexillum) to identify potential symbiotic bacteria. Tunicate-specific bacterial communities were observed for all three species and their tissue specific location was determined by CARD-FISH. Introduction Tunicates and other marine invertebrates are prolific sources of novel natural products for drug discovery (reviewed in Blunt, 2010). Many of these compounds are biosynthesized by a microbial symbiont of the animal, rather than produced by the animal itself (Schmidt, 2010). For example, the anti-cancer drug patellamide, originally isolated from the colonial ascidian Lissoclinum patella, is now known to be produced by an obligate cyanobacterial symbiont, Prochloron didemni (Schmidt, 2005). Research on such microbial symbionts has focused on their potential for overcoming the “supply problem.” Chemical synthesis of natural products is often challenging and expensive, and isolation of sufficient quantities of drug for clinical trials from wild sources may be impossible or environmentally costly. Culture of the microbial symbiont or heterologous expression of the biosynthetic genes offers a relatively economical solution. Although the microbial origin of many tunicate compounds is now well established, relatively little is known about the extent of such symbiotic associations in tunicates and their biological function. Tunicates (or sea squirts) present an interesting system in which to study bacterial/eukaryotic symbiosis as they are deep-branching members of the Phylum Chordata (Passamaneck, 2005 and Buchsbaum, 1948). -

Eurochordata and Cephalochordata of Persian Gulf & Oman See
Subphylum Urochordata : Tunicates Class Ascidacea Class Larvacea Class Thaliacea Subphylum Cephalochordata : Amphioxus Phylum Chordata : 1 - Subphylum Urochordata 2 Subphylum Cephalochordata (Subphylum Urochordata : Tunicates ) Class Ascidiacea Class Larvacea Class Thaliacea (Subphylum Cephalochordata ) Class Ascidiacea Ascidia Kowalevsky Patricia Kott D. lane David George & Gordon Paterson Herdmania momus savigny Herdmania momus kiamanensis Didemnum candidum savigny Phallusia nigra savigny Styela canopus savigny Class Ascidiacea : Order : Aplousobranchia Family : Polyclinidae : Aplidium cf. rubripunctum C.Monniot & F. Monniot 1997 Polyclinum constellatum Savigny, 1816 Family Didemnidae Didemnum candidum Savigny, 1816 Didemnum cf. granulatum Tokioka, 1954 Didemnum obscurum F. Monniot 1969 Didemnum perlucidum F. Monniot, 1983 Didemnum sp. Didemnum yolky C.Monniot & F. Monniot 1997 Diplosoma listerianum Milne Edwards, 1841 Order : Phlebobranchia Family : Ascidiidae Ascidia sp. Phallusia julinea Sluiter, 1915 Phallusia nigra Savigny, 1816 Order : Stolidobranchia Family : Styelidae Botryllus gregalis Sluiter, 1898 Botryllus niger Herdman, 1886 Botryllus sp. Eusynstyela cf. hartmeyeri Michaelsen, 1904 Polyandrocarpa sp. Styela canopus Savigny, 1816 Symplegma bahraini C.Monniot & F. Monniot 1997 Symplegma brakenhielmi Michaelsen, 1904 Family pyuridae Herdmania momus Savigny, 1816 Pyura curvigona Tokioka, 1950 Pyurid sp. Class Larvacea Larva Seymour Sewell Phylum Chordata Subphylum Urochordata * Class Larvacea Order Copelata Family Fritillariidae -

The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom
lopmen ve ta e l B Williamson, Cell Dev Biol 2012, 1:1 D io & l l o l g DOI: 10.4172/2168-9296.1000101 e y C Cell & Developmental Biology ISSN: 2168-9296 Research Article Open Access The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom Abstract The larval transfer hypothesis states that larvae originated as adults in other taxa and their genomes were transferred by hybridization. It contests the view that larvae and corresponding adults evolved from common ancestors. The present paper reviews the life histories of chordates, and it interprets them in terms of the larval transfer hypothesis. It is the first paper to apply the hypothesis to craniates. I claim that the larvae of tunicates were acquired from adult larvaceans, the larvae of lampreys from adult cephalochordates, the larvae of lungfishes from adult craniate tadpoles, and the larvae of ray-finned fishes from other ray-finned fishes in different families. The occurrence of larvae in some fishes and their absence in others is correlated with reproductive behavior. Adult amphibians evolved from adult fishes, but larval amphibians did not evolve from either adult or larval fishes. I submit that [1] early amphibians had no larvae and that several families of urodeles and one subfamily of anurans have retained direct development, [2] the tadpole larvae of anurans and urodeles were acquired separately from different Mesozoic adult tadpoles, and [3] the post-tadpole larvae of salamanders were acquired from adults of other urodeles. Reptiles, birds and mammals probably evolved from amphibians that never acquired larvae. -

1471-2148-9-187.Pdf
BMC Evolutionary Biology BioMed Central Research article Open Access An updated 18S rRNA phylogeny of tunicates based on mixture and secondary structure models Georgia Tsagkogeorga1,2, Xavier Turon3, Russell R Hopcroft4, Marie- Ka Tilak1,2, Tamar Feldstein5, Noa Shenkar5,6, Yossi Loya5, Dorothée Huchon5, Emmanuel JP Douzery1,2 and Frédéric Delsuc*1,2 Address: 1Université Montpellier 2, Institut des Sciences de l'Evolution (UMR 5554), CC064, Place Eugène Bataillon, 34095 Montpellier Cedex 05, France, 2CNRS, Institut des Sciences de l'Evolution (UMR 5554), CC064, Place Eugène Bataillon, 34095 Montpellier Cedex 05, France, 3Centre d'Estudis Avançats de Blanes (CEAB, CSIC), Accés Cala S. Francesc 14, 17300 Blanes (Girona), Spain, 4Institute of Marine Science, University of Alaska Fairbanks, Fairbanks, Alaska, USA, 5Department of Zoology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, 69978, Israel and 6Department of Biology, University of Washington, Seattle WA 98195, USA Email: Georgia Tsagkogeorga - [email protected]; Xavier Turon - [email protected]; Russell R Hopcroft - [email protected]; Marie-Ka Tilak - [email protected]; Tamar Feldstein - [email protected]; Noa Shenkar - [email protected]; Yossi Loya - [email protected]; Dorothée Huchon - [email protected]; Emmanuel JP Douzery - [email protected]; Frédéric Delsuc* - [email protected] * Corresponding author Published: 5 August 2009 Received: 16 October 2008 Accepted: 5 August 2009 BMC Evolutionary Biology 2009, 9:187 doi:10.1186/1471-2148-9-187 This article is available from: http://www.biomedcentral.com/1471-2148/9/187 © 2009 Tsagkogeorga et al; licensee BioMed Central Ltd. -

DEEP SEA LEBANON RESULTS of the 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project
DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project March 2018 DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project Citation: Aguilar, R., García, S., Perry, A.L., Alvarez, H., Blanco, J., Bitar, G. 2018. 2016 Deep-sea Lebanon Expedition: Exploring Submarine Canyons. Oceana, Madrid. 94 p. DOI: 10.31230/osf.io/34cb9 Based on an official request from Lebanon’s Ministry of Environment back in 2013, Oceana has planned and carried out an expedition to survey Lebanese deep-sea canyons and escarpments. Cover: Cerianthus membranaceus © OCEANA All photos are © OCEANA Index 06 Introduction 11 Methods 16 Results 44 Areas 12 Rov surveys 16 Habitat types 44 Tarablus/Batroun 14 Infaunal surveys 16 Coralligenous habitat 44 Jounieh 14 Oceanographic and rhodolith/maërl 45 St. George beds measurements 46 Beirut 19 Sandy bottoms 15 Data analyses 46 Sayniq 15 Collaborations 20 Sandy-muddy bottoms 20 Rocky bottoms 22 Canyon heads 22 Bathyal muds 24 Species 27 Fishes 29 Crustaceans 30 Echinoderms 31 Cnidarians 36 Sponges 38 Molluscs 40 Bryozoans 40 Brachiopods 42 Tunicates 42 Annelids 42 Foraminifera 42 Algae | Deep sea Lebanon OCEANA 47 Human 50 Discussion and 68 Annex 1 85 Annex 2 impacts conclusions 68 Table A1. List of 85 Methodology for 47 Marine litter 51 Main expedition species identified assesing relative 49 Fisheries findings 84 Table A2. List conservation interest of 49 Other observations 52 Key community of threatened types and their species identified survey areas ecological importanc 84 Figure A1. -

Pyrosomes: Enigmatic Marine Inhabitants with an Important Role in the Cabo Verde Ecosystem 4 May 2021
Pyrosomes: Enigmatic marine inhabitants with an important role in the Cabo Verde ecosystem 4 May 2021 submersibles looked at moribund colonies on the seabed or used net catches that generally disrupt species interactions. Furthermore, the aim was to estimate the contribution of these organisms to the local marine carbon cycle. For the eastern Atlantic such information was still largely unknown. "Because we combined underwater observations, sampling and genetic analyses, we were able to gain several new insights into pyrosome ecology," says lead author Vanessa Stenvers, from GEOMAR. During the expedition, the organisms were observed directly with the research Deep-sea shrimp with a pyrosome on the sea floor. submersible JAGO, and also studied via a pelagic Credit: JAGO Team, GEOMAR. towed camera system, PELAGIOS, as well as by net and water sampling. "Our study shows that pyrosomes form an Pyrosomes, named after the Greek words for 'fire important biological substrate in the water column bodies' due their bright bioluminescence, are that other animals use for settlement, shelter and/or pelagic tunicates that spend their entire lives as a food source," explains Vanessa Stenvers. "We swimming in the open ocean. They are made up of have estimated that Pyrosoma atlanticum provides many smaller animals, known as zooids, that sit up to 0.28 m2 of substrate area per square meter of together in a tubular matrix, known as tunic (hence total area during a bloom period. This is a huge the name pelagic tunicates). Because they live in number if you consider that there are little physical the open ocean, they generally go unnoticed. -

Origin of Chordates Part-I
ORIGIN OF CHORDATES Evolution of chordate was one of the most important event in the history of chordate as it was the beginning of evolution of more advanced chordates like bird and mammals. It is the story of origin from a primitive invertebrate like creatures to early chordates. Though first fossil of the first vertebrate the ostrachoderm was discovered from the Ordovician period but it might have originated in late Cambrian period. As early chordates were soft bodied, their fossil records are not preserved. Hence, to trace their ancestry, we have to find out the similarity among different deuterostomes to trace the origin of chordates. Some structural features shared by them such as bilateral symmetry, antero-posterior body axis, triploblastic coelomate condition, etc., may he because of their common ancestry. TIME OF ORIGIN: Late Cambrian period PLACE OF ORIGIN: Fresh water THEORIES OF INVERTEBRATE ANCESTRY OF CHORDATES Several theories have been put forwarded to explain the origin of chordates either directly from some invertebrate group or through the intervention of some protochordate. Almost every invertebrate phylum—Coelenterata, Nemertean, Phoronida, Annelids, Arthropods and Echinodermatashas been suggested. But these theories are far from being satisfactory and convincing and have only a historical value. Only the echinoderm theory has received some acceptance. DIVISION OF BILATERIA The Bilateria is divided into two major divisions (1) Protostomia and (2) Deuterostornia. This division is based on the differences in embryonic and larval developments. Protostomia includes from Annelida to Arthropoda while deuterostomia includes Echinodermata, Pogonophora and Chordate. DEUTEROSTOME LINE OF CHORDATE EVOLUTION Following common features of all Deuterostomes suggests strong evidence of a closer evolutionary relationship between the three principal deuterostome phyla – Echinodermata, Hemichordata and Chordata. -

Ascidiacea (Chordata: Tunicata) of Greece: an Updated Checklist
Biodiversity Data Journal 4: e9273 doi: 10.3897/BDJ.4.e9273 Taxonomic Paper Ascidiacea (Chordata: Tunicata) of Greece: an updated checklist Chryssanthi Antoniadou‡, Vasilis Gerovasileiou§§, Nicolas Bailly ‡ Department of Zoology, School of Biology, Aristotle University of Thessaloniki, Thessaloniki, Greece § Institute of Marine Biology, Biotechnology and Aquaculture, Hellenic Centre for Marine Research, Heraklion, Greece Corresponding author: Chryssanthi Antoniadou ([email protected]) Academic editor: Christos Arvanitidis Received: 18 May 2016 | Accepted: 17 Jul 2016 | Published: 01 Nov 2016 Citation: Antoniadou C, Gerovasileiou V, Bailly N (2016) Ascidiacea (Chordata: Tunicata) of Greece: an updated checklist. Biodiversity Data Journal 4: e9273. https://doi.org/10.3897/BDJ.4.e9273 Abstract Background The checklist of the ascidian fauna (Tunicata: Ascidiacea) of Greece was compiled within the framework of the Greek Taxon Information System (GTIS), an application of the LifeWatchGreece Research Infrastructure (ESFRI) aiming to produce a complete checklist of species recorded from Greece. This checklist was constructed by updating an existing one with the inclusion of recently published records. All the reported species from Greek waters were taxonomically revised and cross-checked with the Ascidiacea World Database. New information The updated checklist of the class Ascidiacea of Greece comprises 75 species, classified in 33 genera, 12 families, and 3 orders. In total, 8 species have been added to the previous species list (4 Aplousobranchia, 2 Phlebobranchia, and 2 Stolidobranchia). Aplousobranchia was the most speciose order, followed by Stolidobranchia. Most species belonged to the families Didemnidae, Polyclinidae, Pyuridae, Ascidiidae, and Styelidae; these 4 families comprise 76% of the Greek ascidian species richness. The present effort revealed the limited taxonomic research effort devoted to the ascidian fauna of Greece, © Antoniadou C et al.