Contrasting Taxonomic Stratification of Microbial Communities in Two Hypersaline Meromictic Lakes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Analysis of Primary and Secondary Production in Lake Kariba in a Changing Climate
AN ANALYSIS OF PRIMARY AND SECONDARY PRODUCTION IN LAKE KARIBA IN A CHANGING CLIMATE MZIME R. NDEBELE-MURISA A thesis submitted in partial fulfillment of the requirements for the degree of Doctor Philosophiae in the Department of Biodiversity and Conservation Biology, University of the Western Cape Supervisor: Prof. Charles Musil Co-Supervisor: Prof. Lincoln Raitt May 2011 An analysis of primary and secondary production in Lake Kariba in a changing climate Mzime Regina Ndebele-Murisa KEYWORDS Climate warming Limnology Primary production Phytoplankton Zooplankton Kapenta production Lake Kariba i Abstract Title: An analysis of primary and secondary production in Lake Kariba in a changing climate M.R. Ndebele-Murisa PhD, Biodiversity and Conservation Biology Department, University of the Western Cape Analysis of temperature, rainfall and evaporation records over a 44-year period spanning the years 1964 to 2008 indicates changes in the climate around Lake Kariba. Mean annual temperatures have increased by approximately 1.5oC, and pan evaporation rates by about 25%, with rainfall having declined by an average of 27.1 mm since 1964 at an average rate of 6.3 mm per decade. At the same time, lake water temperatures, evaporation rates, and water loss from the lake have increased, which have adversely affected lake water levels, nutrient and thermal dynamics. The most prominent influence of the changing climate on Lake Kariba has been a reduction in the lake water levels, averaging 9.5 m over the past two decades. These are associated with increased warming, reduced rainfall and diminished water and therefore nutrient inflow into the lake. The warmer climate has increased temperatures in the upper layers of lake water, the epilimnion, by an overall average of 1.9°C between 1965 and 2009. -

Template for Two-Page Abstracts in Word 97 (PC)
GEOLOGIC MAPPING OF THE LUNAR SOUTH POLE QUADRANGLE (LQ-30). S.C. Mest1,2, D.C. Ber- man1, and N.E. Petro2, 1Planetary Science Institute, 1700 E. Ft. Lowell, Suite 106, Tucson, AZ 85719-2395 ([email protected]); 2Planetary Geodynamics Laboratory, Code 698, NASA GSFC, Greenbelt, MD 20771. Introduction: In this study we use recent image, the surface [7]. Impact craters display morphologies spectral and topographic data to map the geology of the ranging from simple to complex [7-9,24] and most lunar South Pole quadrangle (LQ-30) at 1:2.5M scale contain floor deposits distinct from surrounding mate- [1-7]. The overall objective of this research is to con- rials. Most of these deposits likely consist of impact strain the geologic evolution of LQ-30 (60°-90°S, 0°- melt; however, some deposits, especially on the floors ±180°) with specific emphasis on evaluation of a) the of the larger craters and basins (e.g., Antoniadi), ex- regional effects of impact basin formation, and b) the hibit low albedo and smooth surfaces and may contain spatial distribution of ejecta, in particular resulting mare. Higher albedo deposits tend to contain a higher from formation of the South Pole-Aitken (SPA) basin density of superposed impact craters. and other large basins. Key scientific objectives in- Antoniadi Crater. Antoniadi crater (D=150 km; clude: 1) Determining the geologic history of LQ-30 69.5°S, 172°W) is unique for several reasons. First, and examining the spatial and temporal variability of Antoniadi is the only lunar crater that contains both a geologic processes within the map area. -

Thureborn Et Al 2013
http://www.diva-portal.org This is the published version of a paper published in PLoS ONE. Citation for the original published paper (version of record): Thureborn, P., Lundin, D., Plathan, J., Poole, A., Sjöberg, B. et al. (2013) A Metagenomics Transect into the Deepest Point of the Baltic Sea Reveals Clear Stratification of Microbial Functional Capacities. PLoS ONE, 8(9): e74983 http://dx.doi.org/10.1371/journal.pone.0074983 Access to the published version may require subscription. N.B. When citing this work, cite the original published paper. Permanent link to this version: http://urn.kb.se/resolve?urn=urn:nbn:se:sh:diva-20021 A Metagenomics Transect into the Deepest Point of the Baltic Sea Reveals Clear Stratification of Microbial Functional Capacities Petter Thureborn1,2*., Daniel Lundin1,3,4., Josefin Plathan2., Anthony M. Poole2,5, Britt-Marie Sjo¨ berg2,4, Sara Sjo¨ ling1 1 School of Natural Sciences and Environmental Studies, So¨derto¨rn University, Huddinge, Sweden, 2 Department of Molecular Biology and Functional Genomics, Stockholm University, Stockholm, Sweden, 3 Science for Life Laboratories, Royal Institute of Technology, Solna, Sweden, 4 Department of Biochemistry and Biophysics, Stockholm University, Stockholm, Sweden, 5 School of Biological Sciences, University of Canterbury, Christchurch, New Zealand Abstract The Baltic Sea is characterized by hyposaline surface waters, hypoxic and anoxic deep waters and sediments. These conditions, which in turn lead to a steep oxygen gradient, are particularly evident at Landsort Deep in the Baltic Proper. Given these substantial differences in environmental parameters at Landsort Deep, we performed a metagenomic census spanning surface to sediment to establish whether the microbial communities at this site are as stratified as the physical environment. -

A Dolomitization Event at the Oceanic Chemocline During the Permian-Triassic Transition Mingtao Li1, Haijun Song1*, Thomas J
https://doi.org/10.1130/G45479.1 Manuscript received 11 August 2018 Revised manuscript received 4 October 2018 Manuscript accepted 5 October 2018 © 2018 The Authors. Gold Open Access: This paper is published under the terms of the CC-BY license. Published online 23 October 2018 A dolomitization event at the oceanic chemocline during the Permian-Triassic transition Mingtao Li1, Haijun Song1*, Thomas J. Algeo1,2,3, Paul B. Wignall4, Xu Dai1, and Adam D. Woods5 1State Key Laboratory of Biogeology and Environmental Geology, School of Earth Science, China University of Geosciences, Wuhan 430074, China 2State Key Laboratory of Geological Processes and Mineral Resources, School of Earth Science, China University of Geosciences, Wuhan 430074, China 3Department of Geology, University of Cincinnati, Cincinnati, Ohio 45221-0013, USA 4School of Earth and Environment, University of Leeds, Leeds LS2 9JT, UK 5Department of Geological Sciences, California State University, Fullerton, California 92834-6850, USA ABSTRACT in dolomite in the uppermost Permian–lowermost Triassic was first noted The Permian-Triassic boundary (PTB) crisis caused major short- by Wignall and Twitchett (2002) and has been attributed to an increased term perturbations in ocean chemistry, as recorded by the precipita- weathering flux of Mg-bearing clays to the ocean (Algeo et al., 2011). tion of anachronistic carbonates. Here, we document for the first time Here, we examine the distribution of dolomite in 22 PTB sections, docu- a global dolomitization event during the Permian-Triassic transition menting a global dolomitization event and identifying additional controls based on Mg/(Mg + Ca) data from 22 sections with a global distri- on its formation during the Permian-Triassic transition. -

Water Resources Report
MMINNEAPOLISINNEAPOLIS PPARKARK && RRECREATIONECREATION BBOARDOARD 20122012 WWATERATER RRESOURCESESOURCES RREPORTEPORT Environmental Stewardship Water Resources Management www.minneapolisparks.org January 2015 2012 WATER RESOURCES REPORT Prepared by: Minneapolis Park & Recreation Board Environmental Stewardship 3800 Bryant Avenue South Minneapolis, MN 55409-1029 612.230.6400 www.minneapolisparks.org January 2015 Funding provided by: Minneapolis Park & Recreation Board City of Minneapolis Public Works Copyright © 2015 by the Minneapolis Park & Recreation Board Material may be quoted with attribution. TABLE OF CONTENTS Page Abbreviations ............................................................................................................................. i Executive Summary ............................................................................................................... iv 1. Monitoring Program Overview .............................................................................................. 1-1 2. Birch Pond .............................................................................................................................. 2-1 3. Brownie Lake ......................................................................................................................... 3-1 4. Lake Calhoun ......................................................................................................................... 4-1 5. Cedar Lake ............................................................................................................................ -

Top-Down Trophic Cascades in Three Meromictic Lakes Tanner J
Top-down Trophic Cascades in Three Meromictic Lakes Tanner J. Kraft, Caitlin T. Newman, Michael A. Smith, Bill J. Spohr Ecology 3807, Itasca Biological Station, University of Minnesota Abstract Projections of tropic cascades from a top-down model suggest that biotic characteristics of a lake can be predicted by the presence of planktivorous fish. From the same perspective, the presence of planktivorous fish can theoretically be predicted based off of the sampled biotic factors. Under such theory, the presence of planktivorous fish contributes to low zooplankton abundances, increased zooplankton predator-avoidance techniques, and subsequent growth increases of algae. Lakes without planktivorous fish would theoretically experience zooplankton population booms and subsequent decreased algae growth. These assumptions were used to describe the tropic interactions of Arco, Deming, and Josephine Lakes; three relatively similar meromictic lakes differing primarily from their absence or presence of planktivorous fish. Due to the presence of several other physical, chemical, and environmental factors that were not sampled, these assumptions did not adequately predict the relative abundances of zooplankton and algae in a lake based solely on the fish status. However, the theory did successfully predict the depth preferences of zooplankton based on the presence or absence of fish. Introduction Trophic cascades play a major role in the ecological composition of lakes. One trophic cascade model predicts that the presence or absence of piscivorous fishes will affect the presence of planktivorous fishes, zooplankton size and abundance, algal biomass, and 1 subsequent water clarity (Fig. 1) (Carpenter et al., 1987). The trophic nature of lakes also affects animal behavior, such as the distribution patterns of zooplankton as a predator avoidance technique (Loose and Dawidowicz, 1994). -
Relative Ages
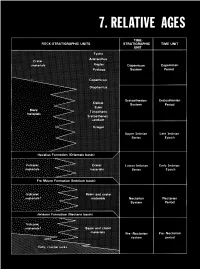
CONTENTS Page Introduction ...................................................... 123 Stratigraphic nomenclature ........................................ 123 Superpositions ................................................... 125 Mare-crater relations .......................................... 125 Crater-crater relations .......................................... 127 Basin-crater relations .......................................... 127 Mapping conventions .......................................... 127 Crater dating .................................................... 129 General principles ............................................. 129 Size-frequency relations ........................................ 129 Morphology of large craters .................................... 129 Morphology of small craters, by Newell J. Fask .................. 131 D, method .................................................... 133 Summary ........................................................ 133 table 7.1). The first three of these sequences, which are older than INTRODUCTION the visible mare materials, are also dominated internally by the The goals of both terrestrial and lunar stratigraphy are to inte- deposits of basins. The fourth (youngest) sequence consists of mare grate geologic units into a stratigraphic column applicable over the and crater materials. This chapter explains the general methods of whole planet and to calibrate this column with absolute ages. The stratigraphic analysis that are employed in the next six chapters first step in reconstructing -

Halanaerobaculum Jean Luc Cayol
Halanaerobaculum Jean Luc Cayol To cite this version: Jean Luc Cayol. Halanaerobaculum. Bergey’s Manual of Systematics of Archaea and Bacteria (BMSAB), Hoboken, New Jersey : Wiley, [2015]-, 2019, 10.1002/9781118960608.gbm01725. hal- 02883868 HAL Id: hal-02883868 https://hal.archives-ouvertes.fr/hal-02883868 Submitted on 29 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 gbm01725 2 3 Halanaerobaculum 4 5 Hedi, Fardeau, Sadfi, Boudabous, Ollivier and Cayol 2009, 317 VP 6 7 Jean-Luc Cayol 8 9 Aix Marseille Université, IRD, Université de Toulon, CNRS, Mediterranean Institute of 10 Oceanography (MIO), UM 110, 13288 Marseille cedex 9, France 11 12 Hal.an.ae.ro.ba’cu.lum. Gr. masc. n. hals, salt; Gr. pref. an, not; Gr. masc. or fem. n. aer, air; 13 L. neut. n. baculum, stick; N.L. neut. n. Halanaerobaculum, salt stick not living in air. 14 15 Abstract 16 The genus Halanaerobaculum comprises one species with validly published name, 17 Halanaerobaculum tunisiense, which was isolated from hypersaline surface sediments of El- 18 Djerid Chott, Tunisia. The genus is halophilic, strict anaerobic and chemoorganotrophic. The 19 genus Halanaerobaculum belongs to the family Halobacteroidaceae; order Halanaerobiales; 20 class Clostridia. -

Town of Seneca
TOWN OF BRISTOL Inventory of Land Use and Land Cover Prepared for: Ontario County Water Resources Council 20 Ontario Street, 3rd Floor Canandaigua, New York 14424 and Town of Bristol 6740 County Road 32 Canandaigua, New York 14424 Prepared by: Dr. Bruce Gilman Department of Environmental Conservation and Horticulture Finger Lakes Community College 3325 Marvin Sands Drive Canandaigua, New York 14424-8395 2020 Cover image: Ground level view of a perched swamp white oak forest community (S1S2) surrounding a shrub swamp that was discovered and documented on Johnson Hill north of Dugway Road. This forest community type is rare statewide and extremely rare locally, and harbors a unique assemblage of uncommon plant species. (Image by the Bruce Gilman). Acknowledgments: For over a decade, the Ontario County Planning Department has supported a working partnership between local towns and the Department of Environmental Conservation and Horticulture at Finger Lakes Community College that involves field research, ground truthing and digital mapping of natural land cover and cultural land use patterns. Previous studies have been completed for the Canandaigua Lake watershed, the southern Honeoye Valley, the Honeoye Lake watershed, the complete Towns of Canandaigua, Gorham, Richmond and Victor, and the woodlots, wetlands and riparian corridors in the Towns of Seneca, Phelps and Geneva. This report summarizes the latest land use/land cover study conducted in the Town of Bristol. The final report would not have been completed without the vital assistance of Terry Saxby of the Ontario County Planning Department. He is gratefully thanked for his assistance with landowner information, his patience as the fieldwork was slowly completed, and his noteworthy help transcribing the field maps to geographic information system (GIS) shape files. -

A Three-Component Microbial Consortium from Deep-Sea Salt-Saturated Anoxic Lake Thetis Links Anaerobic Glycine Betaine Degradation with Methanogenesis
Microorganisms 2015, 3, 500-517; doi:10.3390/microorganisms3030500 OPEN ACCESS microorganisms ISSN 2076-2607 www.mdpi.com/journal/microorganisms Article A Three-Component Microbial Consortium from Deep-Sea Salt-Saturated Anoxic Lake Thetis Links Anaerobic Glycine Betaine Degradation with Methanogenesis Violetta La Cono 1, Erika Arcadi 1, Gina La Spada 1, Davide Barreca 2, Giuseppina Laganà 2, Ersilia Bellocco 2, Maurizio Catalfamo 1, Francesco Smedile 1, Enzo Messina 1, Laura Giuliano 1,3 and Michail M. Yakimov 1,* 1 Institute for Coastal Marine Environment, CNR, Spianata S. Raineri 86, Messina 98122, Italy; E-Mails: [email protected] (V.L.C.); [email protected] (E.A.); [email protected] (G.L.S.); [email protected] (M.C.); [email protected] (F.S.); [email protected] (E.M.); [email protected] (L.G.) 2 Department of Organic and Biological Chemistry, University of Messina, Salita Sperone 31, Villaggio S. Agata, Messina 98166, Italy; E-Mails: [email protected] (D.B.); [email protected] (G.L.); [email protected] (E.B.) 3 Mediterranean Science Commission (CIESM), 16 bd de Suisse, MC 98000, Monaco * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +39-090-6015-437. Academic Editors: Ricardo Amils and Elena González Toril Received: 1 July 2015 / Accepted: 1 September 2015 / Published: 9 September 2015 Abstract: Microbial communities inhabiting the deep-sea salt-saturated anoxic lakes of the Eastern Mediterranean operate under harsh physical-chemical conditions that are incompatible with the lifestyle of common marine microorganisms. -

Table S5. the Information of the Bacteria Annotated in the Soil Community at Species Level
Table S5. The information of the bacteria annotated in the soil community at species level No. Phylum Class Order Family Genus Species The number of contigs Abundance(%) 1 Firmicutes Bacilli Bacillales Bacillaceae Bacillus Bacillus cereus 1749 5.145782459 2 Bacteroidetes Cytophagia Cytophagales Hymenobacteraceae Hymenobacter Hymenobacter sedentarius 1538 4.52499338 3 Gemmatimonadetes Gemmatimonadetes Gemmatimonadales Gemmatimonadaceae Gemmatirosa Gemmatirosa kalamazoonesis 1020 3.000970902 4 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas indica 797 2.344876284 5 Firmicutes Bacilli Lactobacillales Streptococcaceae Lactococcus Lactococcus piscium 542 1.594633558 6 Actinobacteria Thermoleophilia Solirubrobacterales Conexibacteraceae Conexibacter Conexibacter woesei 471 1.385742446 7 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas taxi 430 1.265115184 8 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas wittichii 388 1.141545794 9 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas sp. FARSPH 298 0.876754244 10 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sorangium cellulosum 260 0.764953367 11 Proteobacteria Deltaproteobacteria Myxococcales Polyangiaceae Sorangium Sphingomonas sp. Cra20 260 0.764953367 12 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas panacis 252 0.741416341 -

Article Is Available Gen Availability
Hydrol. Earth Syst. Sci., 23, 1763–1777, 2019 https://doi.org/10.5194/hess-23-1763-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. Oxycline oscillations induced by internal waves in deep Lake Iseo Giulia Valerio1, Marco Pilotti1,4, Maximilian Peter Lau2,3, and Michael Hupfer2 1DICATAM, Università degli Studi di Brescia, via Branze 43, 25123 Brescia, Italy 2Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Müggelseedamm 310, 12587 Berlin, Germany 3Université du Quebec à Montréal (UQAM), Department of Biological Sciences, Montréal, QC H2X 3Y7, Canada 4Civil & Environmental Engineering Department, Tufts University, Medford, MA 02155, USA Correspondence: Giulia Valerio ([email protected]) Received: 22 October 2018 – Discussion started: 23 October 2018 Revised: 20 February 2019 – Accepted: 12 March 2019 – Published: 2 April 2019 Abstract. Lake Iseo is undergoing a dramatic deoxygena- 1 Introduction tion of the hypolimnion, representing an emblematic exam- ple among the deep lakes of the pre-alpine area that are, to a different extent, undergoing reduced deep-water mixing. In Physical processes occurring at the sediment–water inter- the anoxic deep waters, the release and accumulation of re- face of lakes crucially control the fluxes of chemical com- duced substances and phosphorus from the sediments are a pounds across this boundary (Imboden and Wuest, 1995) major concern. Because the hydrodynamics of this lake was with severe implications for water quality. In stratified shown to be dominated by internal waves, in this study we in- lakes, the boundary-layer turbulence is primarily caused by vestigated, for the first time, the role of these oscillatory mo- wind-driven internal wave motions (Imberger, 1998).