1 2.6 Physical Chemistry and Thermal Evolution of Ices at Ganymede 1 C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Template for Two-Page Abstracts in Word 97 (PC)
GEOLOGIC MAPPING OF THE LUNAR SOUTH POLE QUADRANGLE (LQ-30). S.C. Mest1,2, D.C. Ber- man1, and N.E. Petro2, 1Planetary Science Institute, 1700 E. Ft. Lowell, Suite 106, Tucson, AZ 85719-2395 ([email protected]); 2Planetary Geodynamics Laboratory, Code 698, NASA GSFC, Greenbelt, MD 20771. Introduction: In this study we use recent image, the surface [7]. Impact craters display morphologies spectral and topographic data to map the geology of the ranging from simple to complex [7-9,24] and most lunar South Pole quadrangle (LQ-30) at 1:2.5M scale contain floor deposits distinct from surrounding mate- [1-7]. The overall objective of this research is to con- rials. Most of these deposits likely consist of impact strain the geologic evolution of LQ-30 (60°-90°S, 0°- melt; however, some deposits, especially on the floors ±180°) with specific emphasis on evaluation of a) the of the larger craters and basins (e.g., Antoniadi), ex- regional effects of impact basin formation, and b) the hibit low albedo and smooth surfaces and may contain spatial distribution of ejecta, in particular resulting mare. Higher albedo deposits tend to contain a higher from formation of the South Pole-Aitken (SPA) basin density of superposed impact craters. and other large basins. Key scientific objectives in- Antoniadi Crater. Antoniadi crater (D=150 km; clude: 1) Determining the geologic history of LQ-30 69.5°S, 172°W) is unique for several reasons. First, and examining the spatial and temporal variability of Antoniadi is the only lunar crater that contains both a geologic processes within the map area. -
Relative Ages
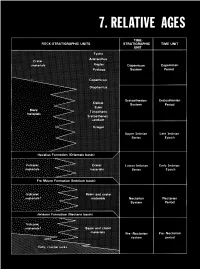
CONTENTS Page Introduction ...................................................... 123 Stratigraphic nomenclature ........................................ 123 Superpositions ................................................... 125 Mare-crater relations .......................................... 125 Crater-crater relations .......................................... 127 Basin-crater relations .......................................... 127 Mapping conventions .......................................... 127 Crater dating .................................................... 129 General principles ............................................. 129 Size-frequency relations ........................................ 129 Morphology of large craters .................................... 129 Morphology of small craters, by Newell J. Fask .................. 131 D, method .................................................... 133 Summary ........................................................ 133 table 7.1). The first three of these sequences, which are older than INTRODUCTION the visible mare materials, are also dominated internally by the The goals of both terrestrial and lunar stratigraphy are to inte- deposits of basins. The fourth (youngest) sequence consists of mare grate geologic units into a stratigraphic column applicable over the and crater materials. This chapter explains the general methods of whole planet and to calibrate this column with absolute ages. The stratigraphic analysis that are employed in the next six chapters first step in reconstructing -

Discoveries of Mass Independent Isotope Effects in the Solar System: Past, Present and Future Mark H
Reviews in Mineralogy & Geochemistry Vol. 86 pp. 35–95, 2021 2 Copyright © Mineralogical Society of America Discoveries of Mass Independent Isotope Effects in the Solar System: Past, Present and Future Mark H. Thiemens Department of Chemistry and Biochemistry University of California San Diego La Jolla, California 92093 USA [email protected] Mang Lin State Key Laboratory of Isotope Geochemistry Guangzhou Institute of Geochemistry, Chinese Academy of Sciences Guangzhou, Guangdong 510640 China University of Chinese Academy of Sciences Beijing 100049 China [email protected] THE BEGINNING OF ISOTOPES Discovery and chemical physics The history of the discovery of stable isotopes and later, their influence of chemical and physical phenomena originates in the 19th century with discovery of radioactivity by Becquerel in 1896 (Becquerel 1896a–g). The discovery catalyzed a range of studies in physics to develop an understanding of the nucleus and the properties influencing its stability and instability that give rise to various decay modes and associated energies. Rutherford and Soddy (1903) later suggested that radioactive change from different types of decay are linked to chemical change. Soddy later found that this is a general phenomenon and radioactive decay of different energies and types are linked to the same element. Soddy (1913) in his paper on intra-atomic charge pinpointed the observations as requiring the observations of the simultaneous character of chemical change from the same position in the periodic chart with radiative emissions required it to be of the same element (same proton number) but differing atomic weight. This is only energetically accommodated by a change in neutrons and it was this paper that the name “isotope” emerges. -

Temperature- and Pressure-Dependent Structural Transformation of Methane Hydrates in Salt Environments
PUBLICATIONS Geophysical Research Letters RESEARCH LETTER Temperature- and pressure-dependent structural 10.1002/2016GL072277 transformation of methane hydrates Key Points: in salt environments • Under a low-pressure condition, the methane hydrate is decomposed Donghoon Shin1 , Minjun Cha2 , Youjeong Yang3, Seunghyun Choi1, Yesol Woo1 , through a rapid sublimation of water 4 5 6 6 7,8,9 1,3 from the surface of hydrate crystals Jong-Won Lee , Docheon Ahn , Junhyuck Im , Yongjae Lee , Oc Hee Han , and Ji-Ho Yoon • The hydrated salt of NaCl · 2H2O 1 undergoes a phase transition into a Department of Energy and Resources Engineering, Korea Maritime and Ocean University, Busan, South Korea, crystal growth of crystalline NaCl via 2Department of Energy and Resources Engineering, Kangwon National University, Chuncheon, South Korea, 3Department the migration of salt ions of Convergence Study on the Ocean Science and Technology, Ocean Science and Technology School, Korea Maritime and • The methane hydrate is fully Ocean University, Busan, South Korea, 4Department of Environmental Engineering, Kongju National University, Chungnam, decomposed in brine solutions at 5 6 temperatures above 252 K, the South Korea, Beamline Department, Pohang Accelerator Laboratory, Pohang, South Korea, Department of Earth System 7 eutectic point of NaCl · 2H2O Sciences, Yonsei University, Seoul, South Korea, Western Seoul Center, Korea Basic Science Institute, Seoul, South Korea, 8Graduate School of Analytical Science and Technology, Chungnam National University, Daejeon, South Korea, 9Department of Chemistry and Nano-Science, College of Natural Sciences, Ewha Womans University, Seoul, South Korea Supporting Information: • Supporting Information S1 Abstract Understanding the stability of volatile species and their compounds under various surface and Correspondence to: J.-H. -

Ethane Clathrate Hydrate Infrared Signatures for Solar System Remote Sensing E Dartois, F Langlet
Ethane clathrate hydrate infrared signatures for solar system remote sensing E Dartois, F Langlet To cite this version: E Dartois, F Langlet. Ethane clathrate hydrate infrared signatures for solar system remote sensing. Icarus, Elsevier, 2021. hal-03159998 HAL Id: hal-03159998 https://hal.archives-ouvertes.fr/hal-03159998 Submitted on 4 Mar 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Ethane clathrate hydrate infrared signatures for solar system remote sensing. E. Dartois1, F. Langlet2, 1 Institut des Sciences Moléculaires d’Orsay, CNRS, Université Paris-Saclay, Bât 520, Rue André Rivière, 91405 Orsay, France e-mail: [email protected] 2 Institut d’Astrophysique Spatiale (IAS), UMR8617, CNRS, Université Paris-Saclay, Bât. 121, 91405 Orsay, France keywords: Planetary ices, IR spectroscopy, Clathrate hydrate, Ethane, Remote sensing To appear in Icarus Abstract their retention time scales in solar system bodies, and also mod- ify their release conditions. b=The hydrate term is sometimes Hydrocarbons such as methane and ethane are present in many used as a semantic shortcut to designate clathrate hydrates but solar system objects, including comets, moons and planets. The must not be confused neither with hydrates nor with ice mixtures interaction of these hydrocarbons with water ice at low temper- where a molecule is interacting with water ice and not necessar- atures could lead to the formation of inclusion compounds, such ily mediated via an ordered crystalline cage. -

Structural and Kinetic Studies of Structure I Gas Hydrates Via Low Temperature X-Ray Diffraction and High Resolution Neutron Diffraction
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2013 Structural and Kinetic Studies of Structure I Gas Hydrates via Low Temperature X-Ray Diffraction and High Resolution Neutron Diffraction Susan Michelle Everett [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Other Materials Science and Engineering Commons Recommended Citation Everett, Susan Michelle, "Structural and Kinetic Studies of Structure I Gas Hydrates via Low Temperature X-Ray Diffraction and High Resolution Neutron Diffraction. " PhD diss., University of Tennessee, 2013. https://trace.tennessee.edu/utk_graddiss/1718 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Susan Michelle Everett entitled "Structural and Kinetic Studies of Structure I Gas Hydrates via Low Temperature X-Ray Diffraction and High Resolution Neutron Diffraction." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Materials Science and Engineering. Claudia -

Thermodynamic Studies on CO2 Capture Through Gas Hydrate Formation Technology
Thermodynamic Studies on CO2 Capture through Gas Hydrate Formation Technology Poorandokht Ilani-Kashkouli MSc. Analytical Chemistry (Shiraz University, Shiraz, Iran, (Sept2009-Jan2012)) This dissertation (ENNO8RPH1) is submitted for the degree of doctorate of philosophy in Engineering (Ph.D. Eng) in the School of Chemical Engineering at the University of KwaZulu- Natal. Supervisor: Prof. Deresh Ramjugernath Co-supervisor(s): Prof. Amir H. Mohammadi, Dr. Paramespri Naidoo April 2015 Declaration I, Poorandokht Ilani-Kashkouli, declare that: i. The research reported in this dissertation, except where otherwise indicated is my original work. ii. This dissertation has not been submitted for any degree or examination at any university. iii. This dissertation does not contain other person’s data, pictures, graphs or other information, unless specifically acknowledged as being sourced from other persons. iv. This dissertation does not contain other person’s writing, unless specifically acknowledged as being sourced from other researchers. Where other written sources have been quoted then: a) Their words have been re-written but the general information attributed to them has been referenced; b) Where their exact words have been used, their writing has been placed inside quotation marks, and referenced. v. Where I have reproduced a publication of which I am an author or co-author I have indicated in detail which part of the publication was actually written by myself alone and have fully referenced such publications. vi. This dissertation does not contain text, graphics or tables copied and pasted from the internet, unless specifically acknowledged, and the source are detailed in the dissertation and in the References sections. -

Carbon Dioxide Clathrate Hydrate Formation at Low Temperature. Diffusion-Limited Kinetics Growth As Monitored by FTIR E
Carbon dioxide clathrate hydrate formation at low temperature. Diffusion-limited kinetics growth as monitored by FTIR E. Dartois1, F. Langlet2, 1 Institut des Sciences Moléculaires d’Orsay, CNRS, Université Paris-Saclay, Bât 520, Rue André Rivière, 91405 Orsay, France e-mail: [email protected] 2 Institut d’Astrophysique Spatiale (IAS), UMR8617, CNRS, Université Paris-Saclay, Bât. 121, 91405 Orsay, France keywords: Infrared: planetary systems; Planets and satellites: composition; Comets: general; Methods: laboratory: solid state; Solid state: volatile; Molecular processes To appear in Astronomy & Astrophysics Abstract tion in climate change if natural fields are destabilised (Riboulot et al. 2018). Carbon dioxide clathrate hydrate is of interest for The formation and presence of clathrate hydrates could influ- fundamental research on Earth, or for its potential use in green- ence the composition and stability of planetary ices and comets; house gas sequestration (Duc et al. 2007; House et al. 2006). they are at the heart of the development of numerous complex planetary models, all of which include the necessary condition In a planetary or astrophysical context, clathrate hydrates imposed by their stability curves, some of which include the are considered because they can retain volatile molecules at cage occupancy or host-guest content and the hydration number, pressures higher than the pure compound that would otherwise but fewer take into account the kinetics aspects. We measure sublimate under most astrophysical conditions, and/or influence the temperature-dependent-diffusion-controlled formation of the the geophysics of large bodies (e.g. Fortes & Choukroun 2010; carbon dioxide clathrate hydrate in the 155-210 K range in or- Choukroun et al. -

Palaeoenvironment in North-Western Romania During the Last 15,000 Years
Palaeoenvironment in north-western Romania during the last 15,000 years Angelica Feurdean Dedicated to Ovidiu Feurdean Avhandling i Kvartärgeologi Thesis in Quaternary Geology No. 3 Department of Physical Geography and Quaternary Geology, Stockholm University 2004 1 ISBN 2 Palaeoenvironment in north-western Romania during the last 15,000 years by Angelica Feurdean Department of Physical Geography and Quaternary Geology, Stockholm University, SE-106 91 Stockholm This thesis is based on work carried out as Ph.D. stu- Appendix III: Feurdean A. (in press). Holocene forest dent at the Department of Paleontology, Faculty of Biol- dynamics in north-western Romania. The Holocene. ogy and Geology, Babes-Bolyai University, Cluj-Napoca, Romania between Oct. 1998 and Sept. 2002 and later as Appendix IV: Feurdean A. & Bennike O. Late Quater- Ph.D. student in Quaternary Geology at the Department nary palaeocological and paleoclimatological reconstruc- of Physical Geography and Quaternary Geology, tion in the Gutaiului Mountains, NW Romania. Manu- Stockholm University 2002-2004. The thesis consists of script submitted to Journal of Quaternary Science. four papers and a synthesis. The four papers are listed below and presented in Appendices I-IV. Two of the pa- Fieldwork in Romania has been jointly performed with pers have been published (I, II), one is in press (III) and Barbara Wohlfarth and Leif Björkman (Preluca Tiganului, the fourth has been submitted (IV). The thesis summary Steregoiu, Izvoare) and with Bogdan Onac (Creasta presents an account of earlier pollenstratigraphic work Cocosului). I am responsible for the lithostratigraphic done in Romania and the discussion focuses on tree description of all sediment and peat cores, for sub-sam- dynamics during the Lateglacial and Holocene, based pling and laboratory preparation. -

Planetary & Solar System Sciences
EGU General Assembly 2012 EGU General Assembly 2012 Programme Group Programme PS – Planetary & Solar System Sciences Monday, 23 April ........................................................................................................................................................................ 2 PS1.1 ........................................................................................................................................................................................ 2 PS2.2 ........................................................................................................................................................................................ 2 GD1.1/PS2.7 .............................................................................................................................................................................. 3 PS3.3 ........................................................................................................................................................................................ 4 PS5.3/ST6.4 ............................................................................................................................................................................... 4 ST2.4/PS5.4 ............................................................................................................................................................................... 6 Tuesday, 24 April ...................................................................................................................................................................... -
![Arxiv:2008.11207V2 [Astro-Ph.GA] 17 Nov 2020 Milky Way’S “Classical Dwarf” Satellite Galaxies](https://docslib.b-cdn.net/cover/3607/arxiv-2008-11207v2-astro-ph-ga-17-nov-2020-milky-way-s-classical-dwarf-satellite-galaxies-1643607.webp)
Arxiv:2008.11207V2 [Astro-Ph.GA] 17 Nov 2020 Milky Way’S “Classical Dwarf” Satellite Galaxies
Draft version November 19, 2020 Typeset using LATEX twocolumn style in AASTeX63 Ultra-faint dwarfs in a Milky Way context: Introducing the Mint Condition DC Justice League Simulations Elaad Applebaum ,1 Alyson M. Brooks ,1 Charlotte R. Christensen ,2 Ferah Munshi ,3 Thomas R. Quinn,4 Sijing Shen ,5 and Michael Tremmel 6 1Department of Physics and Astronomy, Rutgers, The State University of New Jersey, 136 Frelinghuysen Rd., Piscataway, NJ 08854, USA 2Physics Department, Grinnell College, 1116 Eighth Avenue, Grinnell, IA 50112, USA 3Department of Physics & Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA 4Department of Astronomy, University of Washington, Box 351580, Seattle, WA, 98115, USA 5Institute of Theoretical Astrophysics, University of Oslo, Postboks 1029, 0315 Oslo, Norway 6Physics Department, Yale Center for Astronomy & Astrophysics, PO Box 208120, New Haven, CT 06520, USA ABSTRACT We present results from the \Mint" resolution DC Justice League suite of Milky Way-like zoom-in cosmological simulations, which extend our study of nearby galaxies down into the ultra-faint dwarf (UFD) regime for the first time. The mass resolution of these simulations is the highest ever published for cosmological Milky Way zoom-in simulations run to z = 0, with initial star (dark matter) particle masses of 994 (17900) M , and a force resolution of 87 pc. We study the surrounding dwarfs and UFDs, and find the simulations match the observed dynamical properties of galaxies with −3 < MV < −19, and reproduce the scatter seen in the size-luminosity plane for rh & 200 pc. We predict the vast majority of nearby galaxies will be observable by the Vera Rubin Observatory's co-added Legacy Survey of Space and Time (LSST). -

Clathrate Desalination Plant, Preliminary Research Study
CLATHRATE DESALINATION PLANT PRELIMINARY RESEARCH STUDY BY Richard A. McCormack Richard K. Andersen 8 Thermal Energy Storage, Inc. 6335 Ferris Square, Suite E San Diego, California 92121 Contract No. 1425-3-CR-81-19520 Water Treatment Technology Program Report No. 5 June 1995 U.S. DEPARTMENT OF THE INTERIOR Bureau of Reclamation Technical Service Center Water Treatment Engineering and Research Group Form Approved REPORT DOCUMENTATION PAGE Oh48 No. 0704-0188 0~15 Highway. SUW ‘X4. crlln9ton. V4 22202-4302. and 10 the OfWe 01 Management and Budget. Paperwork Reducteon PrOJecr (07040188). Washington, DC 20503. 1. AGENCY USE ONLY (Leave blank) 2. REPORT DATE 3. REPORT TYPE AND DATES COVERED 5. FUNDING NUMBERS CLATHRATE DESALINATION PLANT PRELIMINARY RESEARCH STUDY Contract No. 1425-3-CR-81-19520 6. AUTH%(S) Richard A. McCormack Richacd K. Andersen I : ! 7. PERFORMING ORGANIZATION NAME(S) AND ADDRESS Thermtil Energy Storage, Inc. 6335 Ferris Square, Suite E San Diego, CA 92121 9. SPONSORING/MONITORING AGENCY NAME(S) AND ADDRESS 10. SPONSORlNG/MONlTORlNG AGENCY REPORT NUMBER Bureau of Reclamation Denver Federal Center Water Treatment PO Box 25007 Technology Program Denver, CO 80225-0007 Report No. 5 11. SUPPLEMENTARY NOTES t 12a. DISTRIBUTION /AVAILABILITY STATEMENT 12b. DISTRIBUTION CODE Available from the National Technical Information Service, I Operations Division, 5285 Port Royal Road, Springfield, 1 Virginia 22161 ! 1 t / t j 13. ABSTRACT (Maxrmvm 200 words) jThis report presents preliminary research, design, and cost estimates for a clathrate 1 ifreeze desalination method and system. A clathrate former is injected through the linner pipe of a submerged pipeline to a predetermined ocean depth at which the ocean itemperature is less than the clathrate forming temperature.