The Selection Mosaic and Diversifying Coevolution Between Crossbills and Lodgepole Pine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Track of the Yellowstone Hot Spot: Volcanism, Faulting, and Uplift
Geological Society of America Memoir 179 1992 Chapter 1 The track of the Yellowstone hot spot: Volcanism, faulting, and uplift Kenneth L. Pierce and Lisa A. Morgan US. Geological Survey, MS 913, Box 25046, Federal Center, Denver, Colorado 80225 ABSTRACT The track of the Yellowstone hot spot is represented by a systematic northeast-trending linear belt of silicic, caldera-forming volcanism that arrived at Yel- lowstone 2 Ma, was near American Falls, Idaho about 10 Ma, and started about 16 Ma near the Nevada-Oregon-Idaho border. From 16 to 10 Ma, particularly 16 to 14 Ma, volcanism was widely dispersed around the inferred hot-spot track in a region that now forms a moderately high volcanic plateau. From 10 to 2 Ma, silicic volcanism migrated N54OE toward Yellowstone at about 3 cm/year, leaving in its wake the topographic and structural depression of the eastern Snake River Plain (SRP). This <lo-Ma hot-spot track has the same rate and direction as that predicted by motion of the North American plate over a thermal plume fixed in the mantle. The eastern SRP is a linear, mountain- bounded, 90-km-wide trench almost entirely(?) floored by calderas that are thinly cov- ered by basalt flows. The current hot-spot position at Yellowstone is spatially related to active faulting and uplift. Basin-and-range faults in the Yellowstone-SRP region are classified into six types based on both recency of offset and height of the associated bedrock escarpment. The distribution of these fault types permits definition of three adjoining belts of faults and a pattern of waxing, culminating, and waning fault activity. -

Paleozoic Rocks in the Black Pine Mountains, Cassia County, Idaho
Paleozoic Rocks in the Black Pine Mountains, Cassia County, Idaho GEOLOGICAL SURVEY BULLETIN 1536 Paleozoic Rocks in the Black Pine Mountains, Cassia County, Idaho By]. FRED SMITH, JR. GEOLOGICAL SURVEY BULLETIN 1536 Descriptions of eleven rock units which range in age from Devonian to Permian, the thickest parts being Early Pennsylvanian to· Early Permian UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON 1983 UNITED STATES DEPARTMENT OF THE INTERIOR JAMES G. WATT, Secretary GEOLOGICAL SURVEY Dallas L. Peck, Director Library of Congress Cataloging in Publication Data Smith, J. Fred (Joe Fred), 1911-1982 Paleowic rocks in the Black Pine Mountains, Cassia County, Idaho. (Geological Survey Bulletin 1536) Bibliography: 36 p. Supt. of Docs. No.: I 19.3: 1. Geology, Stratigraphic-Paleozoic. 2. Geology-Idaho-Black Pine Mountains. I. Title. II. Series. QE75.B9 no. 1536 557.3s [551.7'2'0979639] 81-607193 [QE.654] AACR2 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 CONTENTS Page Abstract . 1 Introduction . 2 Stratigraphy . 7 Rocks south of West Dry Canyon fault . 8 Lower plate . 8 Devonian System . 8 Jefferson Formation . 8 Mississippian and Pennsylvanian Systems . 9 Manning Canyon Shale . 9 Middle plate . 11 Pennsylvanian System . 11 Oquirrh Formation, limestone member . 11 Lower part . 11 Middle part . 13 Upper part . 15 Oquirrh Formation, limestone, sandstone, and quartzite mem- ber . 17 Oquirrh Formation, limestone and dolomite member . 18 Upper plate . 21 Pennsylvanian and Permian Systems . 21 Oquirrh Formation sandstone and siltstone member . 21 Rocks north of West Dry Canyon fault . 23 Plate I . 23 Pennsylvanian and Permian Systems . -
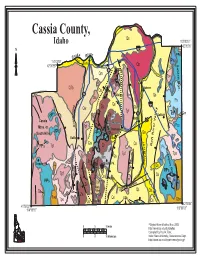
Cassia County, Qs Idaho 113O0010 O Qw 42 3505 86 Qw Ke Iver Ppsp Sna R O Pps 114 0350 Qa Burley O Qb Sublett Rge
Cassia County, Qs Idaho 113o0010 o Qw 42 3505 86 Qw ke iver PPsP Sna R o PPs 114 0350 Qa Burley o Qb Sublett Rge. 42 3055 Declo Qs 77 PPsP Qm PPsP Tov Zs Wm QTb Qa PPs QTb Tpf Idahome PPsP Qs Qs PPsP 27 Albion Tps Qs QTb Ps Qs 84 k Zs e Qw Qs e r Tpf 81 TsR Qw C Zs Sublett P Tpf Wm PPs e Malta Qs s Tov Cassia o Wm o Zs Wm Pzu G Tpf Mtns. or Mt. Harrison South Hills PPsP PPsP PPsP Qa Oakley PzZm Elba Ps Ps Qs Qs r e Pzu Tpf Zs Raft River Valley Ps PPsP Ps Blackpine Rge. v Zs i R Tps Tpf Qs Tpf Wm t PzZm f PPs Tpf Zs Tpf a Trapper Pk. Cache Pk. Tms R Qa Tpf Toi Toi PPsP Tpf Zs Tpf Toi Qs Qs Almo Tpf Ms PPsP Tpf Albion Mtns. Wm PPs Zs Zs Toi PPs PPs Almo Qs Qa PzZm Pluton Raft RiverTpf Narrows. Qa Tpf Zs City of Tms Qs Tps Tpf PPsP Qbo Qs Rocks Tpf Qs o o 42 0004 41 5932 o 114o1655 113 0005 Digital Atlas of Idaho, Nov. 2002 0 5 10 miles http://imnh.isu.edu/digitalatlas Compiled by Paul K. Link, 0 8 16 kilometers Idaho State University, Geosciences Dept. http://www.isu.edu/departments/geology/ Cassia County Cassia County, on the south side of the Snake River forms much of Idahos southern boundary with Utah and Nevada on the west. It contains a diverse assemblage of rocks, including the oldest rocks in Idaho, the metamorphic Green Creek gneiss in the Albion Mountains core complex. -

Ground-Water Possibilities South of the Snake River Between Twin Falls and Pocatello, Idaho
Ground-Water Possibilities South of the Snake River Between Twin Falls and Pocatello, Idaho By E. G. CROSTHWAITE CONTRIBUTIONS TO HYDROLOGY GEOLOGICAL SURVEY WATER-SUPPLY PAPER 1460-C Prepared for the United States Bureau of Reclamation with the cooperation of the Idaho Department of Reclamation UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1957 UNITED STATES DEPARTMENT OF THE INTERIOR FRED A. SEATON, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U. S. Government Printing Office Washington 25, D. C. CONTENTS Pag* Abstract _______________________ ...- _ . _____ - _ 99> Introduction-.... _________________________________________ _____ 100 Purpose and scope of report __ _________________________________ 100 Location of area_________________________________________ _ ____ 101 Previous investigations_____________________ ____________________ 102 Geography __ ___________________________________________________ 103' Surface features___-__-____-________--__-________-__--__-___- _ 108- Drainage. ___-___-__-__---_---_-__-_---______--_---_-____ __ _ 100 Climate.. ____________________ . ______________ 107 Precipitation _ ______________________________ _ _ __ ___ 107 Temperature _ ____________________________________ __ _ _ 109* Evaporation __ ___________________________________ _ ___ _ 110' Development ___ ____________________________________ _ _____ 111 Population __ _____________________________________ ___ __ 111 Agriculture. ___ __ _______________________________ _ _____ 113: Industry -
Idaho Snowmobiling
SNOWMOBILING IN IDAHO IDAHO DEPARTMENT OF PARKS AND RECREATION 2020/2021 1 Your Funds at Work 6 Idaho Snowmobile Laws 9 Safe Travel and Ethics 15 Safety & Education Work atFunds Your 22 Idaho’s Trails and Riding Areas 36 Protect Your Privilege This agency’s programs and activities are operated free from discrimination on the basis of race, color, religion, national origin, gender, age or disability. Anyone who believes they have been discriminated against or who needs further information regarding discrimination should write: P.I.O., Idaho Department of Parks and Recreation, PO BOX 83720, Boise, ID, 83720-0065, Costs associated with this publication are available from the Idaho Department of Parks and Recreation in accordance with Section 60-202, Idaho Code. HB366: 9/20/25u/27614. WHAT ARE THE CERTIFICATE OF NUMBER REQUIREMENTS? n or before November 1 of each year, the O owner of each snowmobile shall file an application for number with the department on forms approved by the Idaho Department of Parks and Recreation (department). The department or its authorized vendor(s) will issue the owner a certificate of number. The certificate of number fee for residents and non-residents is $32.50 ($62.50 for rental machines) and is allocated as follows: • Vendors receive $1.50 for a handling fee. • $1 goes into the statewide snowmobile related search and rescue account. • 15% is utilized by the department for administration and the production of certificate of number stickers. • 85% goes to the county designated by the owner. • All certificate of numbers are valid from November 1 to October 31 of the following year. -

Geology and Mineralization of the Southeastern Part of the Black Pine Mountains, Cassia County, Idaho
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 5-1975 Geology and Mineralization of the Southeastern Part of the Black Pine Mountains, Cassia County, Idaho Don E. French Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Geology Commons Recommended Citation French, Don E., "Geology and Mineralization of the Southeastern Part of the Black Pine Mountains, Cassia County, Idaho" (1975). All Graduate Theses and Dissertations. 1903. https://digitalcommons.usu.edu/etd/1903 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. GEOLOGY AND MINERALIZATION OF THE SOUTHEASTERN PART OF THE BLACK PINE MOUNTAINS, CASSIA COUNTY, IDAHO by Don E. French A thesis submitted in partial fulfillment of requirements for the degree of MASTER OF SCIENCE in Geology Approved: Major Professor Committee Member Committee Member Dean of Graduate Studies UTAH STATE UNIVERSITY Logan, utah 1975 ii ACKNOWLEDGMENTS The author is grateful to Dr. Donald R. Olsen, Dr. Clyde T. Hardy, and Dr. Richard R. Alexander for their assistance in preparation of this report. Helpful comments were also given by Mr. Richard Harris and Mr. Perry West of Newmont Exploration Ltd. and by Mr. Louis Cramer of Salt Lake City, Utah. An important contribution was made by Patricia S. French who provided en couragement and financial and technical assistance. Don E. French iii TABLE OF CONTENTS Page ACKNOWLEDGMENTS · . -

Geography: Topography & Mountains S
Digital Atlas of Idaho Geography: Topography & Mountains Idaho's natural history online Exercise: 1 Instructions: Answer the following questions: 1. Much of the topography in southeast Idaho is the result of Basin and Range faulting, creating successive s mountain ranges and valleys. When did this Basin and Range faulting begin? 2. What is believed to be the total lateral extension undergone by southeast Idaho since Basin and Range fault- ing began? 3. Use the Eastern Idaho drainage & mountain range map and color all the mountain ranges named. Color those that you believe to be a result of Basin and Range faulting a different color than the others. (hint: use Rocks, Rails & Trails maps on page 4 and page 5 to help decide). 4. Those mountains that are not the result of Basin and Range faulting are the result of what? 5. Which mountain(s) have the oldest rocks? (look at maps on page 4 and page 5). 6. Using the Eastern Idaho Geology map label the following places with the corresponding letters: A. Middle Proterozoic rock in the northern Lemhi Range. B. Middle Proterozoic rocks near Borah Peak. C. Archean rocks of the Albion Mountains. D. The closest place in Idaho to the Tetons. E. Late Proterozoic rocks of the Bannock Range. F. Late Proterozoic rocks of the Portneuf Range, near Pebble Creek Ski area. 7. Locate the following geographic and geologic features on the Eastern Idaho drainage & mountain range map, and put the corresponding letter for each in the correct box on the map. Use each letter only one time: A. -
Snowmobiling in Idaho
SNOWMOBILING IN IDAHO IDAHO DEPARTMENT OF PARKS AND RECREATION 2016/2017 1 Your Funds at Work 6 Idaho Snowmobile Laws 9 Safe Travel and Ethics 14 Safety & Education Work atFunds Your 22 Idaho’s Trails and Riding Areas 36 Protect Your Privilege This agency’s programs and activities are operated free from discrimination on the basis of race, color, religion, national origin, gender, age or disability. Anyone who believes they have been discriminated against or who needs further information regarding discrimination should write: P.I.O., Idaho Department of Parks and Recreation, PO BOX 83720, Boise, ID, 83720-0065, Costs associated with this publication are available from the Idaho Department of Parks and Recreation in accordance with Section 60-202, Idaho Code. HB366: 9/16/10u/27614. WHAT ARE THE CERTIFICATE OF NUMBER REQUIREMENTS? n or before November 1 of each year, the O owner of each snowmobile shall file an application for number with the department on forms approved by the Idaho Department of Parks and Recreation (department). The department or its authorized vendor(s) will issue the owner a certificate of number. The certificate of number fee for residents is $32.50 ($62.50 for rental machines) and is allocated as follows: • Vendors receive $1.50 for a handling fee. • $1 goes into the statewide snowmobile related search and rescue account. • 15% is utilized by the department for administration and the production of certificate of number stickers. • 85% goes to the county designated by the owner. • All certificate of numbers are valid from November 1 to October 31 of the following year. -

Geologic Bibliography of Raft River Geothermal Area and Vicinity, Cassia County, Idaho
Geologic Bibliography of Raft River Geothermal Area and Vicinity, Cassia County, Idaho Steven C. Devine Bill Bonnichsen Technical Report 79-2 Idaho Geological Survey 1979 Univcrsity of Idaho Moscow, ID 83844-3014 GEOLOGIC BIBLIOGRAPHY OF RAPT RIVER GEOTHERMAL AREA AND VICINITY, CASSIA COUNTY, IDAHO Steven C. Devine Bill Bonnichsen Idaho Geological Survey University of Idaho Technical Report 79-2 Moscow, Idaho April 1979 GEOLOGIC BIBLIOGRAPHY OF RAFT RIVER GEOTHERMAL AREA AND VICINITY, CASSIA COUNTY, IDAHO by 1 Steven C. Devine and 1 Bill Bonnichsen Ackerman, H. D., 1975, Velocity sections in Raft River, Idaho, geothermal area from seismic refractions: U. S. Geological Survey Open-File Report 78-106. Anderson, A. L., 1931a, Geology and mineral resources of eastern Cassia County, Idaho: University of Chicago Ph.D. dissertation. --------, 1931b, Geology and mineral resources of eastern Cassia County, Idaho: Idaho Bureau of Mines and Geology Bulletin 14, p. 49-59. --------, 1934, Contact phenomena associated with the Cassia batholith, Idaho: Journal of Geology, v. 42, p. 376-392. Anderson, W. L., 1977, Interpretation of electromagnetic soundings in the Raft River geothermal area, Idaho: U. S. Geological Survey Open-File Report 77-557. Armstrong, F. C. and S. S. Oriel, 1965, Tectonic development of the Idaho-Wyoming thrust belt: American Association of Petroleum Geologists Bulletin, v. 49, p. 1847-1866. Armstrong, R. L.} 1963, Geochronology and geology of the eastern Great Basin in Nevada and Utah: Yale University Ph,D. dissertation. lIdaho Bureau of Mines and Geology, Moscow, Idaho 83843. 2 -------- 1966, Pre-Tertiary stratigraphy of the Albion Range, southern Idaho (abstract): Geological Society of America Program, 1966, Annual Meeting, p. -

Geochemistry and Geochronology of the Jim Sage
Cenozoic Tectonics, Magmatism, and Stratigraphy of the Snake River Plain– Yellowstone Region and Adjacent Areas themed issue Geochemistry and geochronology of the Jim Sage volcanic suite, southern Idaho: Implications for Snake River Plain magmatism and its role in the history of Basin and Range extension Alexandros Konstantinou1,*, John Valley2, Ariel Strickland2, Elizabeth L. Miller1, Chris Fisher3, Jeffrey Ver- voort3, and Joseph Wooden1,4 1Department of Geological and Environmental Sciences, Stanford University, Stanford, California 94305, USA 2Department of Geoscience, University of Wisconsin, 1215 W. Dayton Street, Madison, Wisconsin 53706, USA 3School of the Environment, Washington State University, Pullman, Washington 99164-2812, USA 4U.S. Geological Survey, Menlo Park, California 94025, USA ABSTRACT (without the large calderas inferred for the Bryan, 2007), yet their origin is one of the SRP rhyolites) implies that there might be fundamental debates in igneous petrology and The Jim Sage volcanic suite (JSVS) an alternative mechanism for the formation geodynamics. This debate is long standing due exposed in the Jim Sage and Cotterel Moun- of the low-δ18O signature other than the pro- to the complex nature of how large silicic mag- tains of southern Idaho (USA) consists of two posed assimilation of hydrothermally altered matic systems are generated and magma stored volcanic members composed of ~240 km3 caldera blocks. One suggestion is that the for large periods of time in heterogeneous con- of Miocene rhyolite lavas separated by an north to south propagation of SRP-type low- tinental crust. Understanding the origin and interval of lacustrine sediments. It is capped δ18O rhyolitic melt along the Albion fault led longevity of large silicic complexes is of great by rheomorphic ignimbrite and as much as to off-axis magmatism. -

Geologic Map Compilation of the Malad City 30 X 60 Minute Quadrangle, Idaho Sean P
IDAHO GEOLOGICAL SURVEY TECHNICAL REPORT 07-1 MOSCOW-BOISE-POCATELLO LONG AND LINK Project supported in 2006 by NSF EAR #0331174, to Paul Link. Map compiled by spl (6/06) redrafted and edited by pkl (9/06) This Technical Report is a reproduction of independent compilation by Sean P. Long and Paul K. Link of Idaho State University, Pocatello, This map has not been externally edited. The authors made Idaho. Its content and format may not conform to agency standards. minimal changes to map units and fault locations. Please address any comments to Paul Link [email protected] Geologic Map Compilation of the Malad City 30 x 60 Minute Quadrangle, Idaho Sean P. Long and Paul K. Link 2007 Salt Lake Formation, tuff (Pliocene? to Upper Miocene) Includes several Oquirrh Group unit b (Late to Early? Pennsylvanian) Interbedded sandy Brigham Group, undifferentiated (Lower Cambrian to Neoproterozoic) Shown Kellogg, K.S., Harlan, S.S., Mehnert, H.H., Snee, L.W., Pierce, K.L., Hackett, W.R., Description of Map Units Tt tuff units mapped in Sublett and Deep Creek mountains; includes white to gray, PobI to silty limestone and calcareous sandstone to siltstone, with rare bioclastic CZb only in northeast corner of map area, includes strata of the Camelback Mountain adn Rodgers, D.W., 1994, Major 10.2 Ma rhyolitic volcanism in the eastern vitric air-fall tuff and water-lain tuff, pink to gray vitric ash-flow tuff, pink to limestone; lithology identical to unit c, 1600-2750 ft (490-840 m) thick. Quartzite, Mutual Formation, Inkom Formation, Caddy Canyon Quartzite, and Papoose Snake rvier Plain, Idaho--Isotopic age and stratigrpahic setting of the Arbon Map units were generally taken from previous geologic maps; some units were gray, rhyolitic welded tuffs, and a 10.4-10.8 Ma (K-Ar age) (Armstrong et al, Creek Formation, with combined thickness of 4300 ft (1310 m). -
Geology and Mineral Resources of Eastern Cassia County, Idaho
STATE OF IDAHO, .· C. BEN Ross, Governor IDAHO BUREAU OF MINES AND GEOLOGY Moscow, Idaho JOHN w. FINCH, Secretary IDAHO BUREAU OF MINES AND GEOLOGY BULLETIN PRICE 50 CENTS No.14 September, 1931 GEOLOGY AND MINERAL RESOURCES OF EASTERN CASSIA COUNTY, IDAHO by ALFRED L. ANDERSON UNIVERSITY OF IDAHO MOSCOW, IDAHO Entered as second class matter Aug. 11, 1924, at the postoffice at Moscow, Idaho, under the Act of March 3, 1879 STATE OF IDAHO C. BEN Ross, Governor IDAHO BUREAU OF MINES AND GEOLOGY Moscow, Idaho JOHN w. FINCH, Secretary IDAHO BUREAU OF MINES AND GEOLOGY BULLETIN PRICE 50 CENTS No.14 September, 1931 GEOLOGY AND MINERAL RESOURCES OF EASTERN CASSIA COUNTY, IDAHO by ALFRED L. ANDERSON UNIVERSITY OF IDAHO MOSCOW, IDAHO Entered as second class matter Aug. 11, 1924, at the postoffice at Moscow, Idaho, under the Act of March 3, 1879 CONTENTS Page Abstract. vii Introduction ........................................................ 1 Purpose and scope of the investigation .............................. 1 Fieldwork ....................................................... 3 Acknowledgments ................................................ 4 Previous geologic work ............................................ 4 Geography ............................................... ·.......... 7 Location ........................................................ 7 Topography ..................................................... 7 Physiographic setting .......................................... 7 Mountains ...................................................