Abdominoinguinal Incision for the Resection of Pelvic Tumors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Practice Guideline for Limb Salvage Or Early Amputation
Limb Salvage or Early Amputation Evidence-Based Clinical Practice Guideline Adopted by: The American Academy of Orthopaedic Surgeons Board of Directors December 6, 2019 Endorsed by: Please cite this guideline as: American Academy of Orthopaedic Surgeons. Limb Salvage or Early Amputation Evidence-Based Clinical Practice Guideline. https://www.aaos.org/globalassets/quality-and-practice-resources/dod/ lsa-cpg-final-draft-12-10-19.pdf Published December 6, 2019 View background material via the LSA CPG eAppendix Disclaimer This clinical practice guideline was developed by a physician volunteer clinical practice guideline development group based on a formal systematic review of the available scientific and clinical information and accepted approaches to treatment and/or diagnosis. This clinical practice guideline is not intended to be a fixed protocol, as some patients may require more or less treatment or different means of diagnosis. Clinical patients may not necessarily be the same as those found in a clinical trial. Patient care and treatment should always be based on a clinician’s independent medical judgment, given the individual patient’s specific clinical circumstances. Disclosure Requirement In accordance with AAOS policy, all individuals whose names appear as authors or contributors to this clinical practice guideline filed a disclosure statement as part of the submission process. All panel members provided full disclosure of potential conflicts of interest prior to voting on the recommendations contained within this clinical practice guideline. Funding Source This clinical practice guideline was funded exclusively through a research grant provided by the United States Department of Defense with no funding from outside commercial sources to support the development of this document. -

En Bloc Resection of Extra-Peritoneal Soft Tissue Neoplasms Incorporating a Type III Internal Hemipelvectomy: a Novel Approach Sanjay S Reddy1* and Norman D Bloom2
Reddy and Bloom World Journal of Surgical Oncology 2012, 10:222 http://www.wjso.com/content/10/1/222 WORLD JOURNAL OF SURGICAL ONCOLOGY REVIEW Open Access En bloc resection of extra-peritoneal soft tissue neoplasms incorporating a type III internal hemipelvectomy: a novel approach Sanjay S Reddy1* and Norman D Bloom2 Abstract Background: A type III hemipelvectomy has been utilized for the resection of tumors arising from the superior or inferior pubic rami. Methods: In eight patients, we incorporated a type III internal hemipelvectomy to achieve an en bloc R0 resection for tumors extending through the obturator foramen or into the ischiorectal fossa. The pelvic ring was reconstructed utilizing marlex mesh. This allowed for pelvic stability and abdominal wall reconstruction with obliteration of the obturator space to prevent herniations. Results: All eight patients had an R0 resection with an overall survival of 88% and with average follow up of 9.5 years. Functional evaluation utilizing the Enneking classification system, which evaluates motion, pain, stability and strength of the affected extremity, revealed a 62% excellent result and a 37% good result. No significant complications were associated with the operative procedure. Marlex mesh reconstruction provided pelvic stability and eliminated all hernial defects. Conclusion: The superior and inferior pubic rami provide a barrier to a resection for tumors that arise in the extra-peritoneal pelvis extending through the obturator foramen or ischiorectal fossa. Incorporating a type III internal -

Use of Anterolateral Thigh Flap for Reconstruction of Traumatic Bilateral Hemipelvectomy After Major Pelvic Trauma
Al‑wageeh et al. surg case rep (2020) 6:247 https://doi.org/10.1186/s40792‑020‑01009‑2 CASE REPORT Open Access Use of anterolateral thigh fap for reconstruction of traumatic bilateral hemipelvectomy after major pelvic trauma: a case report Saleh Al‑wageeh1 , Faisal Ahmed2* , Khalil Al‑naggar3 , Mohammad Reza Askarpour4 and Ebrahim Al‑shami5 Abstract Background: Major pelvic trauma (MPT) with traumatic hemipelvectomy (THP) is rare, but it is a catastrophic health problem caused by high‑energy injury leading to separation of the lower extremity from the axial skeleton, which is associated with a high incidence of intra‑abdominal and multi‑systemic injuries. THP is generally performed as a lifesaving protocol to return the patient to an active life. Case report: A 12‑year male patient exposed to major pelvic trauma with bilateral THP survived the trauma and mul‑ tiple lifesaving operations. The anterolateral thigh fap is the method used for wound reconstruction. The follow‑up was ended with colostomy and cystostomy with wheelchair mobilization. To the best of our knowledge, there have been a few bilateral THP reports, and our case is the second one to be successfully treated with an anterolateral thigh fap. Conclusion: MPT with THP is the primary cause of death among trauma patients. Life‑threatening hemorrhage is the usual cause of death, which is a strong indication for THP to save life. Keywords: Amputation, Hemipelvectomy, Myocutaneous fap, Reconstruction, Trauma Introduction A few victims survive these injuries, and the actual Major pelvic trauma (MPT) associated with traumatic incidence is unknown, but it is usually underestimated hemipelvectomy (THP) was described frst by Turnbull in [3]. -
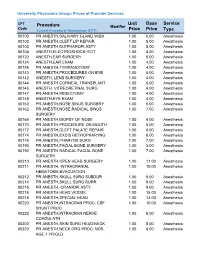
Unit Price Base Price Service Type Procedure
University Physicians Group: Prices of Provider Services CPT Unit Base Service Procedure Modifier Code ype Current Procedural Terminology (CPT) Price Price T 00100 PR ANESTH,SALIVARY GLAND W/BX 1.00 5.00 Anesthesia 00102 PR ANESTH,CLEFT LIP REPAIR 1.00 6.00 Anesthesia 00103 PR ANESTH,BLEPHAROPLASTY 1.00 5.00 Anesthesia 00104 ANESTH,ELECTROSHOCK/ ECT 1.00 4.00 Anesthesia 00120 ANESTH,EAR SURGERY 1.00 5.00 Anesthesia 00124 ANESTH,EAR EXAM 1.00 4.00 Anesthesia 00126 PR ANESTH,TYMPANOTOMY 1.00 4.00 Anesthesia 00140 PR ANESTH,PROCEDURES ON EYE 1.00 5.00 Anesthesia 00142 ANESTH, LENS SURGERY 1.00 4.00 Anesthesia 00144 PR ANESTH,CORNEAL TRANSPLANT 1.00 6.00 Anesthesia 00145 ANESTH, VITREORETINAL SURG 1.00 6.00 Anesthesia 00147 PR ANESTH,IRIDECTOMY 1.00 4.00 Anesthesia 00148 ANESTH,EYE EXAM 1.00 4.00 Anesthesia 00160 PR ANESTH,NOSE,SINUS SURGERY 1.00 5.00 Anesthesia 00162 PR ANESTH,NOSE,RADICAL SINUS 1.00 7.00 Anesthesia SURGERY 00164 PR ANESTH,BIOPSY OF NOSE 1.00 4.00 Anesthesia 00170 PR ANESTH,PROCEDURE ON MOUTH 1.00 5.00 Anesthesia 00172 PR ANESTH,CLEFT PALATE REPAIR 1.00 6.00 Anesthesia 00174 PR ANESTH,EXCIS RETROPHARYNG 1.00 6.00 Anesthesia 00176 PR ANESTH,PHARYNX SURG 1.00 7.00 Anesthesia 00190 PR ANESTH,FACIAL BONE SURGERY 1.00 5.00 Anesthesia 00192 PR ANESTH,RADICAL FACIAL BONE 1.00 7.00 Anesthesia SURGERY 00210 PR ANESTH,OPEN HEAD SURGERY 1.00 11.00 Anesthesia 00211 PR ANESTH, INTRACRANIAL 1.00 10.00 Anesthesia HEMATOMA EVACUATION 00212 PR ANESTH,SKULL SURG SUBDUR 1.00 5.00 Anesthesia 00214 PR ANESTH,SKULL SURG BURR 1.00 9.00 Anesthesia -

Electromyography and Nerve Conduction Studies in Patients With
ogy & N ol eu ur e ro N p h f y o s l Ziegler MS, J Neurol Neurophysiol 2014, 5:3 i o a l n o r g u y o DOI: 10.4172/2155-9562.1000203 J Journal of Neurology & Neurophysiology ISSN: 2155-9562 Research Article Open Access Electromyography and Nerve Conduction Studies in Patients with Lumbar Spinal Stenosis: Is Neurophysiological Examination an Important Tool? Marcus Sofia Ziegler1, Renata Siciliani Scalco2, Erasmo de Abreu Zardo1, Jefferson Becker2 and Irenio Gomes2* 1Department of Orthopaedics & Spine Surgery, Hospital São Lucas Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil 2Department of Neurology, Hospital São Lucas Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil *Corresponding author: Irenio Gomes, Hospital Sao Lucas, and Avenida Ipiranga 6690 - 3 ºandar - IGG Porto Alegre - RS – Brasil, CEP 90610-000, Tel: +55 (51) 3336.8153 / 3320.3000; E-mail: [email protected] Received Date: Jan 21, 2014 Accepted Date: Apr 25, 2014 Published Date: Apr 30, 2014 Copyright: © 2014 Ziegler MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Background: There is no single test that defines properly lumbar spinal stenosis (LSS) diagnosis, and diagnosis of the syndrome continues to rely on clinical judgment. LSS symptoms may be broad and may be seen in multiple disorders in elderly. Hypothesis: To identify the role of electromyography and nerve-conduction studies on LSS diagnosis. -
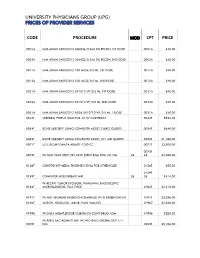
Code Procedure Cpt Price University Physicians Group
UNIVERSITY PHYSICIANS GROUP (UPG) PRICES OF PROVIDER SERVICES CODE PROCEDURE MOD CPT PRICE 0001A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 1ST DOSE 0001A $40.00 0002A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 2ND DOSE 0002A $40.00 0011A IMM ADMN SARSCOV2 100 MCG/0.5 ML 1ST DOSE 0011A $40.00 0012A IMM ADMN SARSCOV2 100 MCG/0.5 ML 2ND DOSE 0012A $40.00 0021A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 1ST DOSE 0021A $40.00 0022A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 2ND DOSE 0022A $40.00 0031A IMM ADMN SARSCOV2 AD26 5X10^10 VP/0.5 ML 1 DOSE 0031A $40.00 0042T CEREBRAL PERFUS ANALYSIS, CT W/CONTRAST 0042T $954.00 0054T BONE SURGERY USING COMPUTER ASSIST, FLURO GUIDED 0054T $640.00 0055T BONE SURGERY USING COMPUTER ASSIST, CT/ MRI GUIDED 0055T $1,188.00 0071T U/S LEIOMYOMATA ABLATE <200 CC 0071T $2,500.00 0075T 0075T PR TCAT PLMT XTRC VRT CRTD STENT RS&I PRQ 1ST VSL 26 26 $2,208.00 0126T CAROTID INT-MEDIA THICKNESS EVAL FOR ATHERSCLER 0126T $55.00 0159T 0159T COMPUTER AIDED BREAST MRI 26 26 $314.00 PR RECTAL TUMOR EXCISION, TRANSANAL ENDOSCOPIC 0184T MICROSURGICAL, FULL THICK 0184T $2,315.00 0191T PR ANT SEGMENT INSERTION DRAINAGE W/O RESERVOIR INT 0191T $2,396.00 01967 ANESTH, NEURAXIAL LABOR, PLAN VAG DEL 01967 $2,500.00 01996 PR DAILY MGMT,EPIDUR/SUBARACH CONT DRUG ADM 01996 $285.00 PR PERQ SAC AGMNTJ UNI W/WO BALO/MCHNL DEV 1/> 0200T NDL 0200T $5,106.00 PR PERQ SAC AGMNTJ BI W/WO BALO/MCHNL DEV 2/> 0201T NDLS 0201T $9,446.00 PR INJECT PLATELET RICH PLASMA W/IMG 0232T HARVEST/PREPARATOIN 0232T $1,509.00 0234T PR TRANSLUMINAL PERIPHERAL ATHERECTOMY, RENAL -

Why Electromyoneurography Instead Of
Neurological Disorders and Therapeutics Research Article ISSN: 2514-4790 Why electromyoneurography instead of electromyography and electroneurography? Electroneurography is essential for interpretation of electromyographic results! Anica Jušić* Shool of Medicine University of Zagreb, Zagreb, 10000 Zagreb, Gundulićeva 49, Croatia Abstract The author suggests revival and further development of old method who had proven to be of significant benefit in differential dignostics of nerve lesions and displayed significant research possibilities. The basic idea is the unification of electromyographic results with neural stimulation. Therefore the author suggests again, to use new name - Electromyoneurography for the old methods. Introduction electrodes as I learned at Albrecht Struppler’s laboratory. The stimulation may be done with surface electrodes also, but the reliability, George Bernard Shaw once wrote: „The single biggest problem constancy and reproducibility of the results, with such technique, in communication is the illusion that it has taken place“. I hope this decreases significantly. With needle electrodes, by simple switching introductory part will make possible, among nowadays achieved of the polarity, besides the efferent motor conduction velocity the scientific circumstances, usage and further development of methods additional afferent conduction velocity measurements can be done. If differentiated some decades ago in Centre /Institute for neuromuscular necessary, the ribbon electrodes for percutaneous evocation of sensory disease, of University Hospital Clinic Zagreb, which I have founded, potentials may be involved. 1973. The purpose of this review is to shed the light on the old methods who had proven to be of significant benefit in differential dignosis of Analyses of evoked muscle or nerve potentials clarifyes so many nerve lesions and possible basis for further scientific research. -

Cross-Sectional Imaging in the Evaluation of Osteogenic Sarcoma: MRI and CT
Cross-sectional Imaging in the Evaluation of Osteogenic Sarcoma: MRI and CT By Leanne L. Seeger, Jeffrey J. Eckardt, and Lawrence W. Bassett EFORE the advent of cross-sectional scan- for treatment. Magnetic resonance imaging and B ning techniques, imaging of malignant bone CT image acquisition should address specific tumors was confined to plain radiography and issues that are important to the surgeon and radionuclide bone scanning. While radiography should be tailored according to tumor location remains the primary imaging modality for differ- and proposed surgical treatment, whether it be ential diagnosis, magnetic resonance imaging amputation or resection with limb salvage proce- (MRI) and computed tomography (CT) have dure. had a profound impact on the preoperative stag- The following discussion reflects the scanning ing evaluation of bone tumors and their response principles and techniques we use for evaluating to therapy. patients with OGS both preoperatively and post- In this article we will define the role of MRI operatively. This was derived from both our own and CT in the work-up of the patient with known experience and the experience of others as re- osteogenic sarcoma (OGS), stressing imaging ported in the literature. ‘-’ Regional differences in strategies that optimize information available to the management of patients with malignant bone the clinician and assist in therapy planning. In tumors may alter this procedure. To become order to achieve these optimal factors, communi- familiar with local treatment protocols, commu- cation with the referring physician and review of nicate directly with the surgeon who will ulti- available radiographs and radionuclide bone scans mately be responsible for treatment. -

ICD-9-CM Procedures (FY10)
2 PREFACE This sixth edition of the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) is being published by the United States Government in recognition of its responsibility to promulgate this classification throughout the United States for morbidity coding. The International Classification of Diseases, 9th Revision, published by the World Health Organization (WHO) is the foundation of the ICD-9-CM and continues to be the classification employed in cause-of-death coding in the United States. The ICD-9-CM is completely comparable with the ICD-9. The WHO Collaborating Center for Classification of Diseases in North America serves as liaison between the international obligations for comparable classifications and the national health data needs of the United States. The ICD-9-CM is recommended for use in all clinical settings but is required for reporting diagnoses and diseases to all U.S. Public Health Service and the Centers for Medicare & Medicaid Services (formerly the Health Care Financing Administration) programs. Guidance in the use of this classification can be found in the section "Guidance in the Use of ICD-9-CM." ICD-9-CM extensions, interpretations, modifications, addenda, or errata other than those approved by the U.S. Public Health Service and the Centers for Medicare & Medicaid Services are not to be considered official and should not be utilized. Continuous maintenance of the ICD-9- CM is the responsibility of the Federal Government. However, because the ICD-9-CM represents the best in contemporary thinking of clinicians, nosologists, epidemiologists, and statisticians from both public and private sectors, no future modifications will be considered without extensive advice from the appropriate representatives of all major users. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -
FLEXOR TENOTOMY: a Simplified Technique
CHAPTER 1 FLEXOR TENOTOMY: A Simplified Technique Mickey D. Stapp, DPM Craig Camasta, DPM INTRODUCTION The key to choosing this procedure is that the digital deformity must be flexible or semi-rigid at the interpha - Tenotomies have been performed in foot and ankle langeal joint level and no contracture or a reducible surgeries for many years. Traditionally, open tenotomies deformity at the metatarsophalangeal joint level. This were performed alone, in significant tendon contractures procedure cannot serve as an alternative for an arthrodesis without osseous involvement, or in combination with or arthroplasty of the digit or a full sequential release at the osseous surgery when osseous changes were also present. metatarsophalangeal joint. A percutaneous tenotomy for Many foot and ankle surgeons have understood the flexible digital deformities would be rarely indicated for importance of tenotomies in successful digital surgeries. 1-4 multiple adjacent digits. It is most often utilized on third McGowan may have been first to describe a minimally and fourth toes. invasive technique for tenotomies. 5 The lesion pattern, hyperkeratotic, preulcerative, or Surgeons searching for less invasive procedures to full ulcer, must be taken into consideration. The majority address tendon pathology began utilizing percutaneous of lesions best amenable to this procedure are lesions tenotomies for a multitude of various foot and ankle located at the distal aspect of the digit. This procedure deformities. The vast majority of these have been described provides a simplified technique to eliminate painful distal for clubfoot deformities. 6-8 Prior to the use of percutaneous clavi or recurring ulcerative lesions (Figure 1). tenotomies in clubfoot surgery, this technique was described for various Achilles tendonopathies. -

The Hypotonic Infant: Clinical Approach
Journal of Pediatric Neurology 5 (2007) 181–187 181 IOS Press Review Article The hypotonic infant: Clinical approach Mohammed M.S. Jan∗ Department of Pediatrics, King Abdulaziz University Hospital, and Department of Neurosciences, King Faisal Specialist Hospital & RC, Jeddah, Saudi Arabia Received 27 November 2006 Revised 25 December 2006 Accepted 31 December 2006 Abstract. Hypotonia in infants can be a confusing clinical presentation leading to inaccurate evaluation and unnecessary investigations. Hypotonia can result from a variety of central or peripheral causes. Therefore, hypotonia is a phenotype of many clinical conditions with variable prognosis. It is important to recognize that hypotonia is not equivalent to weakness. Infants with central causes, such as Down syndrome, may have severe hypotonia with normal muscle strength. Peripheral hypotonia is frequently associated with weakness, which can be predominantly distal in neuropathies or predominantly proximal in myopathies. In general, central hypotonia is much more commonly encountered; however, the prognosis is worst for hypotonia secondary to neuromuscular pathology. The distinction between central and peripheral hypotonia is therefore critical for proper evaluation and management. Stepwise and accurate assessment is very important to reach the correct diagnosis promptly. In this review, I present a concise clinical approach for evaluating the hypotonic infant. Some practical tips and skills are discussed to improve the likelihood of obtaining an accurate diagnosis. Reaching a specific diagnosis is needed for providing appropriate therapy, prognosis, and counseling. Keywords: Infant, child, hypotonia, floppy, examination, approach 1. Introduction rological disorders one of the most difficult aspects of their clinical practice [6–8]. Hypotonia in infants Neurological disorders are common in Saudi Ara- and children can be a confusing clinical presentation, bia accounting for 25–30% of all consultations to pe- which often leads to inaccurate evaluation and unnec- diatrics [1].