Hemoglobin–Oxygen Affinity in High-Altitude Vertebrates: Is There Evidence for an Adaptive Trend? Jay F
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Systematic Relationships and Biogeography of the Tracheophone Suboscines (Aves: Passeriformes)
MOLECULAR PHYLOGENETICS AND EVOLUTION Molecular Phylogenetics and Evolution 23 (2002) 499–512 www.academicpress.com Systematic relationships and biogeography of the tracheophone suboscines (Aves: Passeriformes) Martin Irestedt,a,b,* Jon Fjeldsaa,c Ulf S. Johansson,a,b and Per G.P. Ericsona a Department of Vertebrate Zoology and Molecular Systematics Laboratory, Swedish Museum of Natural History, P.O. Box 50007, SE-104 05 Stockholm, Sweden b Department of Zoology, University of Stockholm, SE-106 91 Stockholm, Sweden c Zoological Museum, University of Copenhagen, Copenhagen, Denmark Received 29 August 2001; received in revised form 17 January 2002 Abstract Based on their highly specialized ‘‘tracheophone’’ syrinx, the avian families Furnariidae (ovenbirds), Dendrocolaptidae (woodcreepers), Formicariidae (ground antbirds), Thamnophilidae (typical antbirds), Rhinocryptidae (tapaculos), and Conop- ophagidae (gnateaters) have long been recognized to constitute a monophyletic group of suboscine passerines. However, the monophyly of these families have been contested and their interrelationships are poorly understood, and this constrains the pos- sibilities for interpreting adaptive tendencies in this very diverse group. In this study we present a higher-level phylogeny and classification for the tracheophone birds based on phylogenetic analyses of sequence data obtained from 32 ingroup taxa. Both mitochondrial (cytochrome b) and nuclear genes (c-myc, RAG-1, and myoglobin) have been sequenced, and more than 3000 bp were subjected to parsimony and maximum-likelihood analyses. The phylogenetic signals in the mitochondrial and nuclear genes were compared and found to be very similar. The results from the analysis of the combined dataset (all genes, but with transitions at third codon positions in the cytochrome b excluded) partly corroborate previous phylogenetic hypotheses, but several novel arrangements were also suggested. -
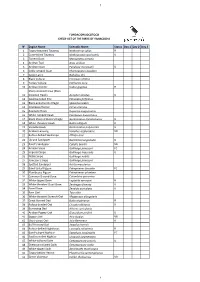
N° English Name Scientific Name Status Day 1
1 FUNDACIÓN JOCOTOCO CHECK-LIST OF THE BIRDS OF YANACOCHA N° English Name Scientific Name Status Day 1 Day 2 Day 3 1 Tawny-breasted Tinamou Nothocercus julius R 2 Curve-billed Tinamou Nothoprocta curvirostris U 3 Torrent Duck Merganetta armata 4 Andean Teal Anas andium 5 Andean Guan Penelope montagnii U 6 Sickle-winged Guan Chamaepetes goudotii 7 Cattle Egret Bubulcus ibis 8 Black Vulture Coragyps atratus 9 Turkey Vulture Cathartes aura 10 Andean Condor Vultur gryphus R Sharp-shinned Hawk (Plain- 11 breasted Hawk) Accipiter striatus U 12 Swallow-tailed Kite Elanoides forficatus 13 Black-and-chestnut Eagle Spizaetus isidori 14 Cinereous Harrier Circus cinereus 15 Roadside Hawk Rupornis magnirostris 16 White-rumped Hawk Parabuteo leucorrhous 17 Black-chested Buzzard-Eagle Geranoaetus melanoleucus U 18 White-throated Hawk Buteo albigula R 19 Variable Hawk Geranoaetus polyosoma U 20 Andean Lapwing Vanellus resplendens VR 21 Rufous-bellied Seedsnipe Attagis gayi 22 Upland Sandpiper Bartramia longicauda R 23 Baird's Sandpiper Calidris bairdii VR 24 Andean Snipe Gallinago jamesoni FC 25 Imperial Snipe Gallinago imperialis U 26 Noble Snipe Gallinago nobilis 27 Jameson's Snipe Gallinago jamesoni 28 Spotted Sandpiper Actitis macularius 29 Band-tailed Pigeon Patagoienas fasciata FC 30 Plumbeous Pigeon Patagioenas plumbea 31 Common Ground-Dove Columbina passerina 32 White-tipped Dove Leptotila verreauxi R 33 White-throated Quail-Dove Zentrygon frenata U 34 Eared Dove Zenaida auriculata U 35 Barn Owl Tyto alba 36 White-throated Screech-Owl Megascops -
![Downloaded from Birdtree.Org [48] to Take Into Account Phylogenetic Uncertainty in the Comparative Analyses [67]](https://docslib.b-cdn.net/cover/5125/downloaded-from-birdtree-org-48-to-take-into-account-phylogenetic-uncertainty-in-the-comparative-analyses-67-245125.webp)
Downloaded from Birdtree.Org [48] to Take Into Account Phylogenetic Uncertainty in the Comparative Analyses [67]
bioRxiv preprint doi: https://doi.org/10.1101/586362; this version posted November 19, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. RESEARCH ARTICLE Open Access Distribution of iridescent colours in Open Peer-Review hummingbird communities results Open Data from the interplay between Open Code selection for camouflage and communication Cite as: preprint Posted: 15th November 2019 Hugo Gruson1, Marianne Elias2, Juan L. Parra3, Christine Recommender: Sébastien Lavergne Andraud4, Serge Berthier5, Claire Doutrelant1, & Doris Reviewers: Gomez1,5 XXX Correspondence: 1 [email protected] CEFE, Univ Montpellier, CNRS, Univ Paul Valéry Montpellier 3, EPHE, IRD, Montpellier, France 2 ISYEB, CNRS, MNHN, Sorbonne Université, EPHE, 45 rue Buffon CP50, Paris, France 3 Grupo de Ecología y Evolución de Vertrebados, Instituto de Biología, Universidad de Antioquia, Medellín, Colombia 4 CRC, MNHN, Ministère de la Culture et de la Communication, CNRS, Paris, France 5 INSP, Sorbonne Université, CNRS, Paris, France This article has been peer-reviewed and recommended by Peer Community In Evolutionary Biology Peer Community In Evolutionary Biology 1 of 33 bioRxiv preprint doi: https://doi.org/10.1101/586362; this version posted November 19, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Identification errors between closely related, co-occurring, species may lead to misdirected social interactions such as costly interbreeding or misdirected aggression. This selects for divergence in traits involved in species identification among co-occurring species, resulting from character displacement. -

Hummingbird (Family Trochilidae) Research: Welfare-Conscious Study Techniques for Live Hummingbirds and Processing of Hummingbird Specimens
Special Publications Museum of Texas Tech University Number xx76 19xx January XXXX 20212010 Hummingbird (Family Trochilidae) Research: Welfare-conscious Study Techniques for Live Hummingbirds and Processing of Hummingbird Specimens Lisa A. Tell, Jenny A. Hazlehurst, Ruta R. Bandivadekar, Jennifer C. Brown, Austin R. Spence, Donald R. Powers, Dalen W. Agnew, Leslie W. Woods, and Andrew Engilis, Jr. Dedications To Sandra Ogletree, who was an exceptional friend and colleague. Her love for family, friends, and birds inspired us all. May her smile and laughter leave a lasting impression of time spent with her and an indelible footprint in our hearts. To my parents, sister, husband, and children. Thank you for all of your love and unconditional support. To my friends and mentors, Drs. Mitchell Bush, Scott Citino, John Pascoe and Bill Lasley. Thank you for your endless encouragement and for always believing in me. ~ Lisa A. Tell Front cover: Photographic images illustrating various aspects of hummingbird research. Images provided courtesy of Don M. Preisler with the exception of the top right image (courtesy of Dr. Lynda Goff). SPECIAL PUBLICATIONS Museum of Texas Tech University Number 76 Hummingbird (Family Trochilidae) Research: Welfare- conscious Study Techniques for Live Hummingbirds and Processing of Hummingbird Specimens Lisa A. Tell, Jenny A. Hazlehurst, Ruta R. Bandivadekar, Jennifer C. Brown, Austin R. Spence, Donald R. Powers, Dalen W. Agnew, Leslie W. Woods, and Andrew Engilis, Jr. Layout and Design: Lisa Bradley Cover Design: Lisa A. Tell and Don M. Preisler Production Editor: Lisa Bradley Copyright 2021, Museum of Texas Tech University This publication is available free of charge in PDF format from the website of the Natural Sciences Research Laboratory, Museum of Texas Tech University (www.depts.ttu.edu/nsrl). -

Abstract Book
Welcome to the Ornithological Congress of the Americas! Puerto Iguazú, Misiones, Argentina, from 8–11 August, 2017 Puerto Iguazú is located in the heart of the interior Atlantic Forest and is the portal to the Iguazú Falls, one of the world’s Seven Natural Wonders and a UNESCO World Heritage Site. The area surrounding Puerto Iguazú, the province of Misiones and neighboring regions of Paraguay and Brazil offers many scenic attractions and natural areas such as Iguazú National Park, and provides unique opportunities for birdwatching. Over 500 species have been recorded, including many Atlantic Forest endemics like the Blue Manakin (Chiroxiphia caudata), the emblem of our congress. This is the first meeting collaboratively organized by the Association of Field Ornithologists, Sociedade Brasileira de Ornitologia and Aves Argentinas, and promises to be an outstanding professional experience for both students and researchers. The congress will feature workshops, symposia, over 400 scientific presentations, 7 internationally renowned plenary speakers, and a celebration of 100 years of Aves Argentinas! Enjoy the book of abstracts! ORGANIZING COMMITTEE CHAIR: Valentina Ferretti, Instituto de Ecología, Genética y Evolución de Buenos Aires (IEGEBA- CONICET) and Association of Field Ornithologists (AFO) Andrés Bosso, Administración de Parques Nacionales (Ministerio de Ambiente y Desarrollo Sustentable) Reed Bowman, Archbold Biological Station and Association of Field Ornithologists (AFO) Gustavo Sebastián Cabanne, División Ornitología, Museo Argentino -

MEMBERSHIP DIRECTORY FORM - from WEBSITE Please Print Clearly and Fill out Completely
APWS MEMBERSHIP DIRECTORY FORM - FROM WEBSITE Please print clearly and fill out completely. Reply ASAP – deadline April 1st ATTENTION: This form MUST be returned to be included in the next Membership Directory! Send completed form to: Lisa Tate, APWS Editor, 21718 Kesa Lane, Florence, MT 59833 PLEASE DO NOT SEND ANY MEMBERSHIP FUNDS WITH THIS FORM REQUIRED - DATE FILLED OUT ______________________________________________ Name __________________________________________________________________________________________________________________________ Aviary Name __________________________________________________________________________________________________________________ Address _______________________________________________________________________________________________________________________ City ________________________________________________________________________ State/Province _________________________________ Country ___________________________________________________________ Zip ________________________________+4___________________ Phone ___________________________________________ Fax ________________________________ Cell __________________________________ Email _________________________________________________________________________________________________________________________ Website ______________________________________________________________________________________________________________________ PLEASE CIRCLE THE NUMBER NEXT TO EACH SPECIES YOU KEEP PHEASANTS (PH) PEACOCK-PHEASANTS 7 - MOUNTAIN BAMBOO JUNGLEFOWL -

Diversity and Natural History of Birds in Green Urban Areas of the City of Quito, Ecuador (America)
Diversity and natural history of birds in green urban areas of the city of Quito, Ecuador (America) Diego F. Cisneros-Heredia1,2,3* & Eliana Montenegro1 1 Universidad San Francisco de Quito, Colegio de Ciencias Biológicas y Ambientales, Laboratorio de Zoología Terrestre. Casilla Postal 17-1200-841, Quito, Ecuador 2 King’s College London, Department of Geography, Strand, London WC2R 2LS, UK 3 Aves&Conservación / BirdLife Ecuador, Quito, Ecuador * E-mail: [email protected] Abstract The diversity and ecology of urban bird communities have been extensively studied in Neartic and Paleartic areas, however, little is known about urban Neotropical areas. Quito, capital city of Ecuador, is located on a highland valley in the megadiverse tropical Andes. Founded in 1534, Quito did not increase significantly its urban area until the late 19th century, growing at an accelerated and unplanned rate during the 20th century. More than 100 species were known to inhabit in Quito at the end of the 19th century. Currently, most authors estimated that no more than 40 species occur in Quito, although no systematic bird studies have been conducted. Our research is a first approach to the avifauna of Quito, surveying the diversity living in green urban areas within the city borders. We used two field methodologies, i.e. line transects and point counts, to survey 16 green urban areas over 12 months. We recorded 65 species of birds, belonging to 20 families and 9 orders. Three species were the most common and frequent: Eared Dove Zenaida auriculata, Rufous-collared Sparrow Zonotrichia capensis, and Great Thrush Turdus fuscater, being omnivores and granivores adapted to anthropic habitats with low ecological complexity. -

Dacninae Species Tree, Part I
Dacninae I: Nemosiini, Conirostrini, & Diglossini Hooded Tanager, Nemosia pileata Cherry-throated Tanager, Nemosia rourei Nemosiini Blue-backed Tanager, Cyanicterus cyanicterus White-capped Tanager, Sericossypha albocristata Scarlet-throated Tanager, Sericossypha loricata Bicolored Conebill, Conirostrum bicolor Pearly-breasted Conebill, Conirostrum margaritae Chestnut-vented Conebill, Conirostrum speciosum Conirostrini White-eared Conebill, Conirostrum leucogenys Capped Conebill, Conirostrum albifrons Giant Conebill, Conirostrum binghami Blue-backed Conebill, Conirostrum sitticolor White-browed Conebill, Conirostrum ferrugineiventre Tamarugo Conebill, Conirostrum tamarugense Rufous-browed Conebill, Conirostrum rufum Cinereous Conebill, Conirostrum cinereum Stripe-tailed Yellow-Finch, Pseudochloris citrina Gray-hooded Sierra Finch, Phrygilus gayi Patagonian Sierra Finch, Phrygilus patagonicus Peruvian Sierra Finch, Phrygilus punensis Black-hooded Sierra Finch, Phrygilus atriceps Gough Finch, Rowettia goughensis White-bridled Finch, Melanodera melanodera Yellow-bridled Finch, Melanodera xanthogramma Inaccessible Island Finch, Nesospiza acunhae Nightingale Island Finch, Nesospiza questi Wilkins’s Finch, Nesospiza wilkinsi Saffron Finch, Sicalis flaveola Grassland Yellow-Finch, Sicalis luteola Orange-fronted Yellow-Finch, Sicalis columbiana Sulphur-throated Finch, Sicalis taczanowskii Bright-rumped Yellow-Finch, Sicalis uropigyalis Citron-headed Yellow-Finch, Sicalis luteocephala Patagonian Yellow-Finch, Sicalis lebruni Greenish Yellow-Finch, -

Bolivia: the Andes and Chaco Lowlands
BOLIVIA: THE ANDES AND CHACO LOWLANDS TRIP REPORT OCTOBER/NOVEMBER 2017 By Eduardo Ormaeche Blue-throated Macaw www.birdingecotours.com [email protected] 2 | T R I P R E P O R T Bolivia, October/November 2017 Bolivia is probably one of the most exciting countries of South America, although one of the less-visited countries by birders due to the remoteness of some birding sites. But with a good birding itinerary and adequate ground logistics it is easy to enjoy the birding and admire the outstanding scenery of this wild country. During our 19-day itinerary we managed to record a list of 505 species, including most of the country and regional endemics expected for this tour. With a list of 22 species of parrots, this is one of the best countries in South America for Psittacidae with species like Blue-throated Macaw and Red-fronted Macaw, both Bolivian endemics. Other interesting species included the flightless Titicaca Grebe, Bolivian Blackbird, Bolivian Earthcreeper, Unicolored Thrush, Red-legged Seriema, Red-faced Guan, Dot-fronted Woodpecker, Olive-crowned Crescentchest, Black-hooded Sunbeam, Giant Hummingbird, White-eared Solitaire, Striated Antthrush, Toco Toucan, Greater Rhea, Brown Tinamou, and Cochabamba Mountain Finch, to name just a few. We started our birding holiday as soon as we arrived at the Viru Viru International Airport in Santa Cruz de la Sierra, birding the grassland habitats around the terminal. Despite the time of the day the airport grasslands provided us with an excellent introduction to Bolivian birds, including Red-winged Tinamou, White-bellied Nothura, Campo Flicker, Chopi Blackbird, Chotoy Spinetail, White Woodpecker, and even Greater Rhea, all during our first afternoon. -

Reference File
References added since publication of 2007 CRC Handbook of Avian Body Masses Abadie, K. B., J. Pérez Z., and M. Valverde. 2006. Primer reporte de colonias del Martín Peruano Progne murphyi. Cotinga 24:99-101. Ackerman, J. T., J. Y. Takekawa, J. D. Bluso, J. L. Yee, and C. A. Eagles-Smith. 2008. Gender identification of Caspian Terns using external morphology and discriminant function analysis. Wilson Journal of Ornithology 120:378-383. Alarcos, S., C. de la Cruz, E. Solís, J. Valencia, and M. J. García-Baquero. 2007. Sex determination of Iberian Azure-winged Magpies Cyanopica cyanus cooki by discriminant analysis of external measurements. Ringing & Migration 23:211-216. Albayrak, T., A. Besnard, and A. Erdoğan. 2011. Morphometric variation and population relationships of Krüeper’s Nuthatch (Sitta krueperi) in Turkey. Wilson Journal of Ornithology 123:734-740. Aleixo, A., C. E. B. Portes, A. Whittaker, J. D. Weckstein, L. Pedreira Gonzaga, K. J. Zimmer, C. C. Ribas, and J. M. Bates. 2013. Molecular systematics and taxonomic revision of the Curve-billed Scythebill complex (Campylorhamphus procurvoides: Dendrocolaptidae), with description of a new species from western Amazonian Brazil. Pp. 253-257, In: del Hoyo, J., A Elliott, J. Sargatal, and D.A. Christie (eds). Handbook of the birds of the world. Special volume: new species and global index. Lynx Edicions, Barcelona, Spain. Volume 1. Alfano, A. 2014. Pygmy Nightjar (Nyctopolus hirundinaeus). Neotropical Birds Online (T.S. Schulenberg, ed.). Cornell Laboratory of Ornithology, Ithaca, NY. Alvarenga, H. M. F., E. Höfling, and L. F. Silveira. 2002. Notharchus swainsoni (Gray, 1846) é uma espécie válida. -

Programs and Field Trips
CONTENTS Welcome from Kathy Martin, NAOC-V Conference Chair ………………………….………………..…...…..………………..….…… 2 Conference Organizers & Committees …………………………………………………………………..…...…………..……………….. 3 - 6 NAOC-V General Information ……………………………………………………………………………………………….…..………….. 6 - 11 Registration & Information .. Council & Business Meetings ……………………………………….……………………..……….………………………………………………………………………………………………………………….…………………………………..…..……...….. 11 6 Workshops ……………………….………….……...………………………………………………………………………………..………..………... 12 Symposia ………………………………….……...……………………………………………………………………………………………………..... 13 Abstracts – Online login information …………………………..……...………….………………………………………….……..……... 13 Presentation Guidelines for Oral and Poster Presentations …...………...………………………………………...……….…... 14 Instructions for Session Chairs .. 15 Additional Social & Special Events…………… ……………………………..………………….………...………………………...…………………………………………………..…………………………………………………….……….……... 15 Student Travel Awards …………………………………………..………...……………….………………………………..…...………... 18 - 20 Postdoctoral Travel Awardees …………………………………..………...………………………………..……………………….………... 20 Student Presentation Award Information ……………………...………...……………………………………..……………………..... 20 Function Schedule …………………………………………………………………………………………..……………………..…………. 22 – 26 Sunday, 12 August Tuesday, 14 August .. .. .. 22 Wednesday, 15 August– ………………………………...…… ………………………………………… ……………..... Thursday, 16 August ……………………………………….…………..………………………………………………………………… …... 23 Friday, 17 August ………………………………………….…………...………………………………………………………………………..... 24 Saturday, -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations.