RESIDENCY RESEARCH PROJECT MANUSCRIPT Evaluating the Utility
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trillium Health Partners Receives Largest Donation in Canadian History
FOR IMMEDIATE RELEASE TRILLIUM HEALTH PARTNERS RECEIVES LARGEST DONATION IN CANADIAN HISTORY FROM MUSLIM COMMUNITY Historic pledge of $5 million to help expand and redevelop Mississauga Hospital Mississauga, On (August 19, 2020) – In a record-breaking pledge, the Muslim community has announced $5 million to support the future redevelopment and expansion of Mississauga Hospital, one of three sites that together comprise Trillium Health Partners (THP), as part of THP’s broader redevelopment plan. Individuals, corporations, mosques and community associations from the Muslim community have a long history of giving back to THP and other health care institutions across the country. Today’s announcement marks the largest gift to a hospital by the Muslim community in Canadian history. Philanthropic support will be an essential component of financing THP’s future redevelopment project. This gift, led and organized by THP Foundation board member Abdul Qayyum Mufti and his closest friends and business associates, Nadeem Chaudhry and Atta Qureshi, shows the Muslim community’s commitment to its faith and the long-term health of the city and the region. “I want to thank Abdul Qayyum Mufti and the Muslim community for showing the true Ontario spirit in making this generous contribution to support our frontline heroes at Trillium Health Partners,” said the Honourable Doug Ford, Premier of Ontario. “Our strength as a province and as a people comes from our diversity and shared values, and it’s needed now more than ever. By having each other’s backs, we can get through these extraordinary times.” “I want to thank Mississauga’s Muslim community for once again stepping up to show how much they care about our city. -

CREDIT VALLEY HOSPITAL CELEBRATES COMPLETION of 20 YEAR EXPANSION PLAN Over 150,000 Square Feet of Renovated Space Improves Care and Experience for Patients
For Immediate Release CREDIT VALLEY HOSPITAL CELEBRATES COMPLETION OF 20 YEAR EXPANSION PLAN Over 150,000 square feet of renovated space improves care and experience for patients November 23, 2018 (Mississauga, ON) – Today, Trillium Health Partners (THP) celebrated the completion of the third phase of its 20 year Credit Valley Hospital (CVH) expansion plan that has added more space to care for more patients. The Honourable Doug Ford, Premier of Ontario, the Honourable Christine Elliott, Deputy Premier and Minister of Health and Long-Term Care, the Honourable Monte McNaughton, Minister of Infrastructure, local Members of Provincial Parliament and community leaders joined front-line staff to mark the momentous occasion with an official ribbon cutting. The CVH Phase 3 Redevelopment Project has improved patient experience, medical technology and created more space to care for the community which includes: Added space that will support care for over 107,000 emergency department visits and 17,000 surgeries annually 187,000 square feet of renovated space and 20,000 square feet of new space for patient care, as well as 12 new or renovated operating rooms; Fully renovated emergency department features additional space to care for patients, as well as dedicated paediatric and mental health treatment areas; New high accuracy computed tomography (CT) scanners that are faster and more accurate; Art installations above each bed in the Intensive Care Unit (ICU) and Critical Care Unit (CCU) to support the healing and wellbeing of patients and their families; and Introduction of robotics assisted surgery. The completion of the CVH Phase 3 Redevelopment Project is the most recent step in THP’s plan to meet the community’s growing need for health care services. -

Trillium Health Partners Celebrates Year of Achievements While
FOR IMMEDIATE RELEASE TRILLIUM HEALTH PARTNERS CELEBRATES YEAR OF ACHIEVEMENTS WHILE WORKING TOGETHER TO FIGHT GLOBAL HEALTH CRISIS Largest hospital in Canada thanks community for help in battling COVID-19 pandemic July 23, 2020 (MISSISSAUGA, ON) –– In the 2019-20 year, Trillium Health Partners (THP) had its busiest, most demanding year yet with a record 1.75 million patient visits, 277,467 visits to the hospital’s Emergency Departments (ED) and Urgent Care Centre, 64,837 surgeries performed and 8,671 babies delivered. We also ended the year fighting COVID-19 on behalf of this community. At THP’s Annual General Meeting, the following achievements were shared from the past year toward creating a new kind of health care for a healthier community: Increasing capacity both inside and outside the hospital to provide more patients with the right care in the right place at the right time; Opening three units at the Reactivation Care Centre in partnership with Humber River Hospital, adding 69 beds of capacity, so patients receive the specialized care and support they need, including occupational therapy, recreational therapy and physiotherapy, to improve their health outcomes and patient experience; Advancing our strategy to transform the way we deliver care by moving forward on plans to expand and modernize our existing sites to meet the community’s health care needs for years to come; Announcing the location of a new health centre in partnership with Heart House Hospice that will improve access to long-term and hospice palliative care for -

PDF of Dixie: Orchards to Industry by Kathleen A. Hicks
Dixie: Orchards to Industry Kathleen A. Hicks DIXIE: ORCHARDS TO INDUSTRY is published by The Friends of the Mississauga Library System 301 Burnhamthorpe Road West, Mississauga, Ontario L5B 3Y3 Canada Copyright © 2006 Mississauga Library System Dixie: Orchards to Industry All rights reserved. II ISBN 0-9697873-8-3 Written by Kathleen A. Hicks Edited by Michael Nix Graphic layout by Joe and Joyce Melito Cover design by Stephen Wahl Front cover photos - The Region of Peel Archives Back cover photo by Stephen Wahl No part of this publication may be produced in any form without the written permission of the Mississauga Library System. Brief passages may be quoted for books, newspaper or magazine articles, crediting the author and title. For photographs contact the source. Extreme care has been taken where copyright of pictures is concerned and if any errors have occurred, the author extends her utmost apology. Care has also been taken with research material. If anyone encounters any discrepancy with the facts contained herein, please send Upper Canada Map (Frederick R. Bercham) your written information to the author in care of the Mississauga Library System. Dixie: Orchards to Industry (Kathleen A. Hicks) (Kathleen Other Books by Kathleen Hicks III The Silverthorns: Ten Generations in America Kathleen Hicks’ V.I.P.s of Mississauga The Life & Times of the Silverthorns of Cherry Hill Clarkson and Its Many Corners Meadowvale: Mills to Millennium Lakeview: A Journey From Yesterday Cooksville: Country to City VIDEO Riverwood: The Estate Dreams Are Made Of Dedication IV dedicate this book to all the people I know and have known who have hailed from Dixie, whom I have shared many inter- esting stories with over the years and have admired tremen- dously for their community dedication: William Teggart, the Kennedys, Dave and Laurie Pallett, Jim McCarthy, Colonel IHarland Sanders, Gord Stanfield, Mildred and Jack Bellegham and Dave Cook to mention a few. -

Medical Oncologist Oncology Program TRILLIUM HEALTH PARTNERS
Medical Oncologist Oncology Program TRILLIUM HEALTH PARTNERS Trillium Health Partners (THP) is the largest community based academic health network in Ontario serving over one million residents in the communities of Mississauga, Peel Region and West Toronto. The hospital encompasses three main sites – Credit Valley Hospital (CVH), Mississauga Hospital (MH), and Queensway Health Centre (QHC) – offering a full range of acute care hospital services, as well as a variety of community-based specialized programs including the Peel Regional Cancer Centre. The Division of Medical Oncology is comprised of a dedicated group of medical oncologists. The Division also has affiliated clinics at Halton Healthcare (Oakville site). The successful applicant will be expected to potentially participate in sub-specialty off site clinics. The Division provides a full spectrum of Oncology consultation and related services. We currently have a clinical position available for a Medical Oncologist to join our team. This position is a fully funded Cancer Care Ontario AFP. Services provided by the Division Medical Oncology include providing MRP coverage for patients admitted to Medical Oncology; providing consultant coverage as required; and participating in shared sub-specialty call and related procedures. The successful candidate will be responsible for cancer treatment and care of patients with a variety of cancers. The successful candidate will promote and exemplify excellence in professional practice and conduct; be licensed or eligible for a license to practice in Ontario; and certified in Medical Oncology by the Royal College of Physicians and Surgeons of Canada. He/she will have evidence of an academic interest and expertise and be eligible for an academic appointment at the University of Toronto (UT), Faculty of Medicine, Department of Medicine. -

Hoodq Detailed Report™ | 145 Cheverie St, Oakville, on L6J 6C3
Michela Mantle 145 Cheverie St 905.330.4077 Oakville, ON michelamantle.com HOODQ DETAILED REPORT™ ELEMENTARY TRANSIT SAFETY SCHOOLS HIGH PARKS CONVENIENCE SCHOOLS PUBLIC SCHOOLS (ASSIGNED) Your neighbourhood is part of a community of Public Schools offering Elementary, Middle, and High School programming. See the closest Public Schools near you below: Maple Grove PS about a 17 minute walk - 1.25 KM away Pre-Kindergarten, Kindergarten, Elementary and Middle 288 Maple Grove Dr, Oakville, ON L6J 4V5, Canada http://mag.hdsb.ca... Address 288 Maple Grove Dr, Oakville, ON L6J 4V5, Canada Language English Grade Level Pre-Kindergarten, Kindergarten, Elementary and Middle School Type Public Phone Number 905-844-9322 School Board Halton DSB School Number 334693 Grades Offered PK to 8 School Board Number B66133 Oakville Trafalgar HS 1.55 KM away High 1460 Devon Rd, Oakville, ON L6J 3L6, Canada Oakville Trafalgar High School, or 'OT' as it is commonly known in the community, started as Oakville High School in 1908 on Reynolds Street. Renamed Oakville Trafalgar High School in the 1940s, the school moved to the present location on Devon Road in 1992. http://oth.hdsb.ca... Address 1460 Devon Rd, Oakville, ON L6J 3L6, Canada Language English Grade Level High School Type Public Phone Number 905-845-2875 School Board Halton DSB School Number 932060 Grades Offered 9 to 12 School Board Number B66133 École EJ James PS 2.03 KM away Elementary and Middle 338 Cairncroft Rd, Oakville, ON L6J 4M6, Canada EJJ is a single track French Immersion school with over 500 students in grades 1 through 8 located in southeast Oakville. -
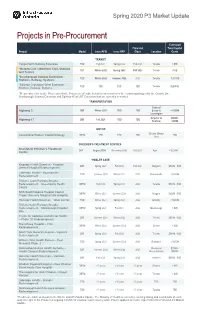
Market Update Spring 2020
Spring 2020 P3 Market Update Projects in Pre-Procurement Estimated Financial Total Capital Project Model Issue RFQ Issue RFP Close Location Costs TRANSIT Yonge North Subway Extension TBD Fall 2021 Spring 2022 Fall 2023 Toronto > $5B *Ontario Line – Northern Civil, Stations DBF Winter 2022 Spring 2022 Fall 2023 Toronto >$3B and Tunnel *Scarborough Subway Extension – TBD Winter 2022 Summer 2022 2023 Toronto $2B-$4B Stations, Railway, Systems *Eglinton Crosstown West Extension - TBD TBD TBD TBD Toronto $2B-$4B Stations, Railway, Systems *Please also refer to the “Projects in Active Procurement” table below for an overview of the contract packages for the Ontario Line, Scarborough Subway Extension and Eglinton West LRT Extension that are currently in-market. TRANSPORTATION Town of Highway 3 DBF Winter 2021 TBD TBD Essex to < $200M Leamington Arnprior to $200M - DBF Fall 2021 TBD TBD Highway 17 Renfrew $499M JUSTICE Greater Ottawa Correctional Eastern Capital Strategy DBFM TBD TBD TBD TBD Area CHILDREN’S TREATMENT CENTRES Grandview Children’s Treatment DBF August 2020 December 2020 Fall 2021 Ajax < $200M Centre *HEALTH CARE Kingston Health Sciences - Kingston DBF Spring 2020 Fall 2020 Fall 2021 Kingston $500M - $1B General Hospital Redevelopment Lakeridge Health – Bowmanville TBD Summer 2020 Winter 2021 2022 Bowmanville < $200M Redevelopment Trillium Health Partners Broader Redevelopment – Queensway Health DBFM Fall 2020 Spring 2021 2022 Toronto $500M - $1B Centre NHS South Niagara Hospital Capital DBFM Winter 2021 Summer 2021 2022 Niagara -

Lakeview: Journey from Yesterday Kathleen A
Lakeview: Journey From Yesterday Kathleen A. Hicks LAKEVIEW: JOURNEY FROM YESTERDAY is published by The Friends of the Mississauga Library System 301 Burnhamthorpe Road, West, Mississauga, Ontario, L5B 3Y3 Copyright © 2005 by the Mississauga Library System All rights reserved Lakeview: Journey From Yesterday ISBN 0-9697873-6-7 II Written by Kathleen A. Hicks Cover design by Stephen Wahl Graphic layout by Joe and Joyce Melito Lakeview Sign by Stephen Wahl Back Cover photo by Stephen Wahl No part of this publication may be produced in any form without the written permission of the Mississauga Library System. Brief passages may be quoted for books, newspaper or magazine articles, crediting the author and title. For photographs contact the source. Extreme care has been taken where copyright of pictures is concerned and if any errors have occurred, the author extends her utmost apology. Care also has been taken with research material. If anyone encounters any discrepancy with the facts contained herein, (Region of Peel Archives) please send your written information to the author in care of the Mississauga Library System. Lakeview: Journey From Yesterday Other Books By Kathleen A. Hicks (Stephen Wahl) III The Silverthorns: Ten Generations in America Kathleen Hicks’ V.I.P.s of Mississauga The Life & Times of the Silverthorns of Cherry Hill Clarkson and its Many Corners Meadowvale: Mills to Millennium VIDEO Riverwood: The Estate Dreams are Made of IV Dedication dedicate this book to my family, the Groveses of Lakeview, where I was born. My grandfather, Thomas Jordan, and my father, Thomas Henry, were instrumental in building many houses and office buildings across southern Ontario. -

Infrastructure Ontario Delivering the Province of Ontario’S Large Infrastructure Projects
Infrastructure Ontario Delivering the Province of Ontario’s large infrastructure projects February 20, 2020 Infrastructure Ontario - Partnering to modernize Ontario’s public assets P3 project pipeline – Subway and transit projects ▶ Ontario Line Subway (DBFM) – Greater than $10 billion ▶ Line 2 East Extension (Scarborough) Subway (TBD) – Greater than $5 billion ▶ Eglinton Crosstown LRT – West Extension (TBD) – Greater than $5 billion ▶ Line 1 Extension (Yonge Street North) Subway (TBD) – Greater than $5 billion ▶ GO Expansion OnCorr (DBFOM) – Greater than $10 billion ▶ GO Expansion Union Station Upgrade – Greater Toronto Platform Expansion (Alliance) Area – $200 – $499 million Infrastructure Ontario - Partnering to modernize Ontario’s public assets 2 Market Update: Transit Projects in Active Procurement Project Model Issue RFQ Issue RFP Financial Location Est. Total Close Capital Costs GO Expansion: GTA Lakeshore East – BF Aug. 2017 April 2018 TBD $200M - 499M Central Corridor GO Expansion: Milton Corridor DBF Nov. 2017 April 2018 TBD GTA $100M - 199M Upgrades GO Expansion: Lakeshore West DBF Dec. 2017 April 2018 TBD GTA $500M - 1B Corridor GO Expansion: DBFOM March 2018 May 2019 2021 GTA > $10B OnCorr QEW Credit River DBF April 2019 Aug. 2019 Sept. 2020 Toronto Bridge $200M - 499M GO Expansion: Lakeshore East BF Feb. 2018 TBD TBD GTA $200M - 499M – West Corridor Infrastructure Ontario - Partnering to modernize Ontario’s public assets 3 Market Update: Subways and Transit in Pre-Procurement Project Model Issue RFQ Issue RFP Financial Location -

48 Bus Time Schedule & Line Route
48 bus time schedule & line map 48 Northbound View In Website Mode The 48 bus line (Northbound) has 2 routes. For regular weekdays, their operation hours are: (1) Northbound: 12:00 AM - 11:25 PM (2) Southbound: 12:03 AM - 11:28 PM Use the Moovit App to ƒnd the closest 48 bus station near you and ƒnd out when is the next 48 bus arriving. Direction: Northbound 48 bus Time Schedule 24 stops Northbound Route Timetable: VIEW LINE SCHEDULE Sunday 12:10 AM - 10:21 PM Monday 5:15 AM - 11:25 PM South Common Centre Bus Terminal Platform E,F 2150 Burnhamthorpe Rd W, Peel Tuesday 12:00 AM - 11:25 PM Erin Mills Pky at Folkway Dr Wednesday 12:00 AM - 11:25 PM Erin Mills Parkway, Peel Thursday 12:00 AM - 11:25 PM Erin Mills Station West Platform 3 Friday 12:00 AM - 11:25 PM 4380 Erin Mills Pky, Peel Saturday 12:00 AM - 11:28 PM Erin Mills Pky North Of Credit Valley Rd Erin Mills Parkway, Mississauga Credit Valley Hospital 2200 Eglinton Ave W, Mississauga 48 bus Info Direction: Northbound Eglinton Ave at Erin Mills Pky Stops: 24 2455 Eglinton Avenue West, Mississauga Trip Duration: 31 min Line Summary: South Common Centre Bus Terminal Erin Mills Town Centre Bus Terminal Platform B Platform E,F, Erin Mills Pky at Folkway Dr, Erin Mills Station West Platform 3, Erin Mills Pky North Of Erin Centre Blvd East Of Mall Access Credit Valley Rd, Credit Valley Hospital, Eglinton Ave at Erin Mills Pky, Erin Mills Town Centre Bus Terminal Erin Mills Pky At Erin Centre Blvd Platform B, Erin Centre Blvd East Of Mall Access, Erin Mills Pky At Erin Centre Blvd, Erin -

Be Informed: Resources for Your Cancer Journey
Be Informed: Resources for Your Cancer Journey Presented by: The Psychosocial and Supportive Care Team HADS and DT Please complete the – Distress Thermometer – Hospital Anxiety and Depression Scale Credit Valley Hospital Mississauga Hospital Queensway Health Centre 2200 Eglinton Avenue West, Mississauga 100 Queensway West, Mississauga 150 Sherway Drive, Toronto 1 Agenda To Identify: • What do I need to know while living with cancer? • Where can I get help? Credit Valley Hospital Mississauga Hospital Queensway Health Centre 2200 Eglinton Avenue West, Mississauga 100 Queensway West, Mississauga 150 Sherway Drive, Toronto What To Expect... Where do I go when I come to How long will I be here? the oncology centre? Do I have to register every time Why do I need to answer so I come? many questionnaires/forms? What are my parking options? Credit Valley Hospital Mississauga Hospital Queensway Health Centre 2200 Eglinton Avenue West, Mississauga 100 Queensway West, Mississauga 150 Sherway Drive, Toronto 2 What To Expect... http://www.youtube.com/watch?v=eyv5Vn2hv0 8 Credit Valley Hospital Mississauga Hospital Queensway Health Centre 2200 Eglinton Avenue West, Mississauga 100 Queensway West, Mississauga 150 Sherway Drive, Toronto What To Expect… ESAS – Computer Station Credit Valley Hospital Mississauga Hospital Queensway Health Centre 2200 Eglinton Avenue West, Mississauga 100 Queensway West, Mississauga 150 Sherway Drive, Toronto 3 What To Expect… ESAS – Paper Version Credit Valley Hospital Mississauga Hospital Queensway Health Centre 2200 Eglinton -

Understanding Medical Assistance in Dying (MAID): the Ontario Experience
Understanding Medical Assistance in Dying (MAID): The Ontario Experience Halton Age Friendly Network November 12, 2020 Dianne Godkin, PhD Senior Ethicist, Trillium Health Partners Investigator, Institute for Better Health Past-President, Canadian Bioethics Society Member, Canadian Association of MAID Assessors and Providers Member, University of Toronto Joint Centre for Bioethics Adjunct Lecturer, Faculty of Nursing, University of Toronto 2 My Role • Advocated for the establishment of a working group to determine Trillium Health Partner’s (THP) approach to medical assistance in dying • Co-chaired working group • Also a member of University of Toronto Joint Centre for Bioethics Task Force on MAID Credit Valley Hospital Mississauga Hospital Queensway Health Centre 3 2200 Eglinton Avenue West, Mississauga 100 Queensway West, Mississauga 150 Sherway Drive, Toronto My Role (cont.) • AID Coordinator at THP from June 2016 to June 2017 – responded to 65 requests & direct oversight of 35 provisions • Continue as Co-chair of AID Oversight Committee • Regularly meet with AID Coordinator(s) • Consult on complex cases Credit Valley Hospital Mississauga Hospital Queensway Health Centre 4 2200 Eglinton Avenue West, Mississauga 100 Queensway West, Mississauga 150 Sherway Drive, Toronto Outline • To provide a timeline of important events related to medical assistance in dying (MAID) • To highlight key aspects of legislation that governs MAID including eligibility requirements • To reflect on MAID experience in Ontario • To discuss other challenges and future considerations Credit Valley Hospital Mississauga Hospital Queensway Health Centre 5 2200 Eglinton Avenue West, Mississauga 100 Queensway West, Mississauga 150 Sherway Drive, Toronto Poll Question #1 6 Time Line 7 In the Beginning… • In 1993, in a 5-4 split decision, the Supreme Court of Canada (SCC) ruled that it was not an individual’s right, but rather the government's right to decide the circumstances of one’s death.