Dhanakumar Final Thesis.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Volume6 Issue8(2)
Volume 6, Issue 8(2), August 2017 International Journal of Multidisciplinary Educational Research Published by Sucharitha Publications 8-43-7/1, Chinna Waltair Visakhapatnam – 530 017 Andhra Pradesh – India Email: [email protected] Website: www.ijmer.in Editorial Board Editor-in-Chief Dr.K. Victor Babu Faculty, Department of Philosophy Andhra University – Visakhapatnam - 530 003 Andhra Pradesh – India EDITORIAL BOARD MEMBERS Prof. S.Mahendra Dev Vice Chancellor Prof. Fidel Gutierrez Vivanco Indira Gandhi Institute of Development Founder and President Research Escuela Virtual de Asesoría Filosófica Mumbai Lima Peru Prof.Y.C. Simhadri Prof. Igor Kondrashin Vice Chancellor, Patna University The Member of The Russian Philosophical Former Director Society Institute of Constitutional and Parliamentary The Russian Humanist Society and Expert of Studies, New Delhi & The UNESCO, Moscow, Russia Formerly Vice Chancellor of Benaras Hindu University, Andhra University Nagarjuna University, Patna University Dr. Zoran Vujisiæ Rector Prof. (Dr.) Sohan Raj Tater St. Gregory Nazianzen Orthodox Institute Universidad Rural de Guatemala, GT, U.S.A Former Vice Chancellor Singhania University, Rajasthan Prof.U.Shameem Prof.K.Sreerama Murty Department of Zoology Andhra University Visakhapatnam Department of Economics Andhra University - Visakhapatnam Dr. N.V.S.Suryanarayana Dept. of Education, A.U. Campus Dr.V.Venkateswarlu Vizianagaram Assistant Professor Dept. of Sociology & Social Work Dr. Kameswara Sharma YVR Acharya Nagarjuna University, Guntur Asst. Professor Dept. of Zoology Prof. P.D.Satya Paul Sri. Venkateswara College, Delhi University, Department of Anthropology Delhi Andhra University – Visakhapatnam I Ketut Donder Prof. Josef HÖCHTL Depasar State Institute of Hindu Dharma Department of Political Economy Indonesia University of Vienna, Vienna & Ex. -

Nagapattinam District 64
COASTAL DISTRICT PROFILES OF TAMIL NADU ENVIS CENTRE Department of Environment Government of Tamil Nadu Prepared by Suganthi Devadason Marine Research Institute No, 44, Beach Road, Tuticorin -628001 Sl.No Contents Page No 1. THIRUVALLUR DISTRICT 1 2. CHENNAI DISTRICT 16 3. KANCHIPURAM DISTRICT 28 4. VILLUPURAM DISTRICT 38 5. CUDDALORE DISTRICT 50 6. NAGAPATTINAM DISTRICT 64 7. THIRUVARUR DISTRICT 83 8. THANJAVUR DISTRICT 93 9. PUDUKOTTAI DISTRICT 109 10. RAMANATHAPURAM DISTRICT 123 11. THOOTHUKUDI DISTRICT 140 12. TIRUNELVELI DISTRICT 153 13. KANYAKUMARI DISTRICT 174 THIRUVALLUR DISTRICT THIRUVALLUR DISTRICT 1. Introduction district in the South, Vellore district in the West, Bay of Bengal in the East and i) Geographical location of the district Andhra Pradesh State in the North. The district spreads over an area of about 3422 Thiruvallur district, a newly formed Sq.km. district bifurcated from the erstwhile Chengalpattu district (on 1st January ii) Administrative profile (taluks / 1997), is located in the North Eastern part of villages) Tamil Nadu between 12°15' and 13°15' North and 79°15' and 80°20' East. The The following image shows the district is surrounded by Kancheepuram administrative profile of the district. Tiruvallur District Map iii) Meteorological information (rainfall / ii) Agriculture and horticulture (crops climate details) cultivated) The climate of the district is moderate The main occupation of the district is agriculture and allied activities. Nearly 47% neither too hot nor too cold but humidity is of the total work force is engaged in the considerable. Both the monsoons occur and agricultural sector. Around 86% of the total in summer heat is considerably mitigated in population is in rural areas engaged in the coastal areas by sea breeze. -
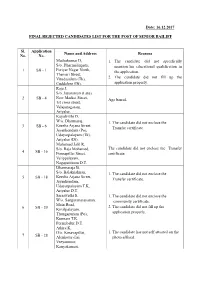
16.12.2017 Final Rejected Candidates List
Date: 16.12.2017 FINAL REJECTED CANDIDATES LIST FOR THE POST OF SENIOR BAILIFF Sl. Application Name and Address Reasons No. No. Muthukumar D, 1. The candidate did not specifically S/o. Dharmalingam, mention his educational qualification in 1 SB - 1 Periyar Nagar North, the application. Thamari Street, Virudasalam (Tk), 2. The candidate did not fill up the Cuddalore (Dt). application properly. Raja J, S/o. Jayaraman (Late), 2 SB - 4 New Market Street, Age barred. 1st cross street, Valajanagaram, Ariyalur. Kayalvizhi D, W/o. Dharmaraj, 1. The candidate did not enclose the Keezha Arjana Street, 3 SB - 6 Transfer certificate. Jayankondam (Po), Udayarpalayam (Tk), Ariyalur (Dt). Muhamad Jalil R, S/o. Raja Mohamed, The candidate did not enclose the Transfer 4 SB - 16 Ponnapillai Street, certificate. Velippalayam, Nagapattinam D.T. Dharmaraja B, S/o. Balakrishnan, 1. The candidate did not enclose the Keezha Arjana Street, 5 SB - 18 Transfer certificate. Jayankondam, Udayarpalayam T.K, Ariyalur D.T. Saraswathi S, 1. The candidate did not enclose the W/o. Sangaranarayanan, community certificate. Main Road, 2. The candidate did not fill up the 6 SB - 20 Kovilpalayam, Thungapuram (Po), application properly. Kunnam T.K. Perambalur D.T. Athira K, D/o. Kesavapillai, 1. The candidate has not self attested on the 7 SB - 28 Alenkottuvilai, photo affixed. Veeyannoor, Kanyakumari. Prasanna J, 1. The candidate did not specially mention S/o. K.Jaganathan, his educational qualification in the 8 SB - 32 Akkrakaram, application. Anathanallur, 2. The candidate did not fill up the Srirangam T.K, application properly. Trichy D.T. Santhi R, W/o. -

Can Farmers Adapt to Climate Change?
CAN FARMERS ADAPT TO CLIMATE CHANGE? CAN FARMERS ADAPT TO CLIMATE CHANGE? Arvind L. Sha J. Jangal R. Suresh CAN FARMERS ADAPT TO CLIMATE CHANGE? Supported by International Development Research Centre under the IDRC Opportunity Fund ISBN 978-818188816-99-x Public Affairs Centre No. 15, KIADB Industrial Area Bommasandra – Jigani Link Road Bangalore -562106 India Phone: +91 80 2783 9918/19/20 Email: [email protected] Web: pacindia.org © 2016 Public Affairs Centre Collaborators and Partners Field Partners Some rights reserved. Content in this publication can be freely shared, distributed, or adapted. However, any work, adapted or otherwise, derived from this publication must be attributed to Public Affairs Centre, Bangalore. This work may not be used for commercial purposes. This book is focussed at livelihood experts, community mangers, Think tanks, NGO’s and academicians who are working to understand the impacts of climate variability and the steps taken by the government, and local bodies to address this issues. This pioneering citizen centric study, triangulates climate change, communities and governance to understand how communities are coping with the issues of climate change. This study was funded as a part of IDRC Opportunity Fund and was conducted in collaboration with CSTEP, and ISET-N. Editing, layout, design and production by PUNYA PUBLISHING PVT. LTD. INDIA ACKNOWLEDGEMENTS This path-breaking study is the first step towards a larger initiative. The study could not have been completed without the help of several individuals and organisations. We are indebted to them, and take this opportunity to thank all those who contributed at various stages of the study. -

The Seasonal Variation of Physic-Chemical Parameters in Surface Water of Kollidam Estuary, Nagapattinam, Se Coast of India
Journal of Xi'an University of Architecture & Technology Issn No : 1006-7930 THE SEASONAL VARIATION OF PHYSIC-CHEMICAL PARAMETERS IN SURFACE WATER OF KOLLIDAM ESTUARY, NAGAPATTINAM, SE COAST OF INDIA RAMESH P*, JAYAPRAKASH M, GOPAL V Dept of Applied Geology, University of Madras, Chennai 600025, India Abstract The physic-chemical properties and enrichment of nutrients were analyzed at 20 stations in Esturine surface water of Kollidam Estuary, Southeast Coast of Tamil Nadu. The analyses were carried out in pre-and post-monsoon of year 2016. The analyzed parameters in premonsoon are pH: 7.2-7.9, DO: 7.9-8.9 mg/l, salinity: 26.8-35.1ppt, PO4: 2.8-6.5 mg/l, Nitirite: 0.12-0.53 mg/l, Nitrate: 26-55 mg/l, silicate: 20.6-42.5 mg/l, Ca: 100-152 mg/l. Mg: 206-356 mg/l, Na: 6.8-12.6g/l and K: 45.3-83.5 mg/l. During postmonsoon, the recorded parameters as pH: 7.9-8.1, DO: 7.6-8.6 mg/l, salinity:27.1-32.7ppt, PO4:4.3-9.36 mg/l, Nitirite: 0.21-0.59 mg/l, Nitrate:33.1-62.9 mg/l, silicate: 24.3-47.1 mg/l, Ca: 64-90 mg/l. Mg: 181-374 mg/l, Na: 76.2-111.1 g/l and K: 9.4-29.7 mg/l. Keywords: Kollidam estuary, Physico-chemical parameters Introduction Coastal water bodies are complex and dynamic in nature due to the complex hydrodynamic process (Morris, et al., 1995). -

3.Hindu Websites Sorted Country Wise
Hindu Websites sorted Country wise Sl. Reference Country Broad catergory Website Address Description No. 1 Afghanistan Dynasty http://en.wikipedia.org/wiki/Hindushahi Hindu Shahi Dynasty Afghanistan, Pakistan 2 Afghanistan Dynasty http://en.wikipedia.org/wiki/Jayapala King Jayapala -Hindu Shahi Dynasty Afghanistan, Pakistan 3 Afghanistan Dynasty http://www.afghanhindu.com/history.asp The Hindu Shahi Dynasty (870 C.E. - 1015 C.E.) 4 Afghanistan History http://hindutemples- Hindu Roots of Afghanistan whthappendtothem.blogspot.com/ (Gandhar pradesh) 5 Afghanistan History http://www.hindunet.org/hindu_history/mode Hindu Kush rn/hindu_kush.html 6 Afghanistan Information http://afghanhindu.wordpress.com/ Afghan Hindus 7 Afghanistan Information http://afghanhindusandsikhs.yuku.com/ Hindus of Afaganistan 8 Afghanistan Information http://www.afghanhindu.com/vedic.asp Afghanistan and It's Vedic Culture 9 Afghanistan Information http://www.afghanhindu.de.vu/ Hindus of Afaganistan 10 Afghanistan Organisation http://www.afghanhindu.info/ Afghan Hindus 11 Afghanistan Organisation http://www.asamai.com/ Afghan Hindu Asociation 12 Afghanistan Temple http://en.wikipedia.org/wiki/Hindu_Temples_ Hindu Temples of Kabul of_Kabul 13 Afghanistan Temples Database http://www.athithy.com/index.php?module=p Hindu Temples of Afaganistan luspoints&id=851&action=pluspoint&title=H indu%20Temples%20in%20Afghanistan%20. html 14 Argentina Ayurveda http://www.augurhostel.com/ Augur Hostel Yoga & Ayurveda 15 Argentina Festival http://www.indembarg.org.ar/en/ Festival of -

Mapping Encroachments Along the River Kaveri in Tamil Nadu, Thanjavur District, India
Available online a twww.scholarsresearchlibrary.com Scholars Research Library Archives of Applied Science Research, 2016, 8 (5):70-76 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-508X CODEN (USA) AASRC9 Flood control measures: mapping encroachments along the river Kaveri in Tamil Nadu, Thanjavur District, India J. Senthil * and P. H. Anand Department of Geography, Govt. Arts College (A), Kumbakonam, Tamil Nadu, India _____________________________________________________________________________________________ ABSTRACT Problem of encroachments on the river Kaveri area is grave and affects the rural environment of this region during seasonal and flash floods. Like the problem of polluted waters, that of encroachments is quite common with almost all the rivers and streams. The first type of encroachment comes from the builders of houses and other concerns. Due to the steep growth of population in Kaveri delta area, and concentration of more rural villages are being affected. The area on the banks of the Kaveri which generally lies vacant and unused seems to be an ideal place for constructing houses and other encroachments. Thus unauthorized colonies and houses are being built on the banks of the Kaveri. Second type of encroachment is by the farmers who own lands adjoining to the Kaveri. The greed for more yields and more income at any cost has led the farmers to hunt for more land. For the gross root level study and to use the GPS survey the entire river length (77 km) has been divided into five zones and identified the encroachments. Key words: Encroachments, GPS, Flash Flood _____________________________________________________________________________________________ INTRODUCTION An illegal intrusion in a highway or navigable river, with or without obstruction is encroachments. -
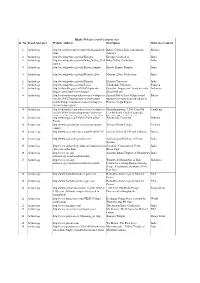
2.Hindu Websites Sorted Category Wise
Hindu Websites sorted Category wise Sl. No. Broad catergory Website Address Description Reference Country 1 Archaelogy http://aryaculture.tripod.com/vedicdharma/id10. India's Cultural Link with Ancient Mexico html America 2 Archaelogy http://en.wikipedia.org/wiki/Harappa Harappa Civilisation India 3 Archaelogy http://en.wikipedia.org/wiki/Indus_Valley_Civil Indus Valley Civilisation India ization 4 Archaelogy http://en.wikipedia.org/wiki/Kiradu_temples Kiradu Barmer Temples India 5 Archaelogy http://en.wikipedia.org/wiki/Mohenjo_Daro Mohenjo_Daro Civilisation India 6 Archaelogy http://en.wikipedia.org/wiki/Nalanda Nalanda University India 7 Archaelogy http://en.wikipedia.org/wiki/Taxila Takshashila University Pakistan 8 Archaelogy http://selians.blogspot.in/2010/01/ganesha- Ganesha, ‘lingga yoni’ found at newly Indonesia lingga-yoni-found-at-newly.html discovered site 9 Archaelogy http://vedicarcheologicaldiscoveries.wordpress.c Ancient Idol of Lord Vishnu found Russia om/2012/05/27/ancient-idol-of-lord-vishnu- during excavation in an old village in found-during-excavation-in-an-old-village-in- Russia’s Volga Region russias-volga-region/ 10 Archaelogy http://vedicarcheologicaldiscoveries.wordpress.c Mahendraparvata, 1,200-Year-Old Cambodia om/2013/06/15/mahendraparvata-1200-year- Lost Medieval City In Cambodia, old-lost-medieval-city-in-cambodia-unearthed- Unearthed By Archaeologists 11 Archaelogy http://wikimapia.org/7359843/Takshashila- Takshashila University Pakistan Taxila 12 Archaelogy http://www.agamahindu.com/vietnam-hindu- Vietnam -

Cif,Ct{6{Dfi (A) Whether the Government Proposes to Develop New Waterways on Rivers and If So, the Details Thereof , Riverl Waterways-Wise;
GOVERNMENT OF INDIA MINISTRY OF SHIPPING LOK SABHA UNSTARRED QUESTION NO. 441 TO BE ANSWERED ON 1gth JULY, 2018 DEVELOPMENT OF WATERWAYS 441. SHRI MANSUKHBHAI DHANJIBHAI VASAVA: SHRI HARISHCHANDRA CHAVAN: DR. RAMESH POKHRIYAL "NISHANK'': Will the Minister of SHIPPING be pleased to state: cIf,ct{6{dfi (a) whether the Government proposes to develop new waterways on rivers and if so, the details thereof , riverl waterways-wise; (b) the details of waterways which are operational in the country, river/ watenrvays-wise; (c) the present status of the National Waterways along with their navigability status, wateruays-wise; (d) whether the Government has established any mechanism for regular monitoring and evaluation of navigability of such waterways and if so, the details thereof; and (e) the details of watenrtrays being used regularly for transportation in the country as on date? ANSWER MINISTER OF STATE IN THE MINISTRY OF SHIPPING (SHRI PON. RADHAKRISHNAN) (a) to (e) To create a country wide waterway network so as to optimize the full potential of this mode of transport, 111 inland waterways (including the existing 5 national waterways) have been declared as National Waterways (NWs) under the 'National Wateruays Act, 2016' which has been enforced w.e.l. 12.04.2016. The list of these NWs is at Annex-|. Subsequent to the declaration of a National Wateruray, feasibility study which inter-alia covers the potential of navigability, cargo availability, cost of development etc. on the NW is undertaken by the lnland WateMays Authority of lndia (lWAl). The details of NWs which are operational/ navigable and being used for transportation at present in the country are at Annex-ll. -

Mint Building S.O Chennai TAMIL NADU
pincode officename districtname statename 600001 Flower Bazaar S.O Chennai TAMIL NADU 600001 Chennai G.P.O. Chennai TAMIL NADU 600001 Govt Stanley Hospital S.O Chennai TAMIL NADU 600001 Mannady S.O (Chennai) Chennai TAMIL NADU 600001 Mint Building S.O Chennai TAMIL NADU 600001 Sowcarpet S.O Chennai TAMIL NADU 600002 Anna Road H.O Chennai TAMIL NADU 600002 Chintadripet S.O Chennai TAMIL NADU 600002 Madras Electricity System S.O Chennai TAMIL NADU 600003 Park Town H.O Chennai TAMIL NADU 600003 Edapalayam S.O Chennai TAMIL NADU 600003 Madras Medical College S.O Chennai TAMIL NADU 600003 Ripon Buildings S.O Chennai TAMIL NADU 600004 Mandaveli S.O Chennai TAMIL NADU 600004 Vivekananda College Madras S.O Chennai TAMIL NADU 600004 Mylapore H.O Chennai TAMIL NADU 600005 Tiruvallikkeni S.O Chennai TAMIL NADU 600005 Chepauk S.O Chennai TAMIL NADU 600005 Madras University S.O Chennai TAMIL NADU 600005 Parthasarathy Koil S.O Chennai TAMIL NADU 600006 Greams Road S.O Chennai TAMIL NADU 600006 DPI S.O Chennai TAMIL NADU 600006 Shastri Bhavan S.O Chennai TAMIL NADU 600006 Teynampet West S.O Chennai TAMIL NADU 600007 Vepery S.O Chennai TAMIL NADU 600008 Ethiraj Salai S.O Chennai TAMIL NADU 600008 Egmore S.O Chennai TAMIL NADU 600008 Egmore ND S.O Chennai TAMIL NADU 600009 Fort St George S.O Chennai TAMIL NADU 600010 Kilpauk S.O Chennai TAMIL NADU 600010 Kilpauk Medical College S.O Chennai TAMIL NADU 600011 Perambur S.O Chennai TAMIL NADU 600011 Perambur North S.O Chennai TAMIL NADU 600011 Sembiam S.O Chennai TAMIL NADU 600012 Perambur Barracks S.O Chennai -
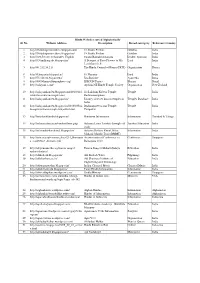
1.Hindu Websites Sorted Alphabetically
Hindu Websites sorted Alphabetically Sl. No. Website Address Description Broad catergory Reference Country 1 http://18shaktipeetasofdevi.blogspot.com/ 18 Shakti Peethas Goddess India 2 http://18shaktipeetasofdevi.blogspot.in/ 18 Shakti Peethas Goddess India 3 http://199.59.148.11/Gurudev_English Swami Ramakrishnanada Leader- Spiritual India 4 http://330milliongods.blogspot.in/ A Bouquet of Rose Flowers to My Lord India Lord Ganesh Ji 5 http://41.212.34.21/ The Hindu Council of Kenya (HCK) Organisation Kenya 6 http://63nayanar.blogspot.in/ 63 Nayanar Lord India 7 http://75.126.84.8/ayurveda/ Jiva Institute Ayurveda India 8 http://8000drumsoftheprophecy.org/ ISKCON Payers Bhajan Brazil 9 http://aalayam.co.nz/ Ayalam NZ Hindu Temple Society Organisation New Zealand 10 http://aalayamkanden.blogspot.com/2010/11/s Sri Lakshmi Kubera Temple, Temple India ri-lakshmi-kubera-temple.html Rathinamangalam 11 http://aalayamkanden.blogspot.in/ Journey of lesser known temples in Temples Database India India 12 http://aalayamkanden.blogspot.in/2010/10/bra Brahmapureeswarar Temple, Temple India hmapureeswarar-temple-tirupattur.html Tirupattur 13 http://accidentalhindu.blogspot.in/ Hinduism Information Information Trinidad & Tobago 14 http://acharya.iitm.ac.in/sanskrit/tutor.php Acharya Learn Sanskrit through self Sanskrit Education India study 15 http://acharyakishorekunal.blogspot.in/ Acharya Kishore Kunal, Bihar Information India Mahavir Mandir Trust (BMMT) 16 http://acm.org.sg/resource_docs/214_Ramayan An international Conference on Conference Singapore -

U.Vinothini, 1/65, Cinnakuruvadi, Irulneekki, Mannargudi Tk
Page 1 of 108 CANDIDATE NAME SL.NO APP.NO AND ADDRESS U.VINOTHINI, 1/65, CINNAKURUVADI, IRULNEEKKI, 1 7511 MANNARGUDI TK,- THIRUVARUR-614018 P.SUBRAMANIYAN, S/O.PERIYASAMY, PERUMPANDI VILL, 2 7512 VANCHINAPURAM PO, ARIYALUR-0 T.MUNIYASAMY, 973 ARIYATHIDAL, POOKKOLLAI, ANNALAKRAHARAM, 3 7513 SAKKOTTAI PO, KUMBAKONAM , THANJAVUR-612401. R.ANANDBABU, S/O RAYAPPAN, 15.AROCKIYAMATHA KOVIL 4 7514 2ND CROSS, NANJIKKOTTAI, THANJAVUR-613005 S.KARIKALAN, S/O.SELLAMUTHU, AMIRTHARAYANPET, 5 7515 SOLAMANDEVI PO, UDAYARPALAYAM TK ARIYALUR-612902 P.JAYAKANTHAN, S/O P.PADMANABAN, 4/C VATTIPILLAIYAR KOILST, 6 7516 KUMBAKONAM THANJAVUR-612001 P.PAZHANI, S/O T.PACKIRISAMY 6/65 EAST ST, 7 7517 PORAVACHERI, SIKKAL PO, NAGAPATTINAM-0 S.SELVANAYAGAM, SANNATHI ST 8 7518 KOTTUR &PO MANNARGUDI TK THIRUVARUR- 614708 Page 2 of 108 CANDIDATE NAME SL.NO APP.NO AND ADDRESS JAYARAJ.N, S/O.NAGARAJ MAIN ROAD, 9 7519 THUDARIPETKALIYAPPANALLUR, TARANGAMPADI TK, NAGAPATTINAM- 609301 K.PALANIVEL, S/O KANNIYAN, SETHINIPURAM ROAD ST, 10 7520 VIKIRAPANDIYAM, KUDAVASAL , THIRUVARUR-610107 MURUGAIYAN.K, MAIN ROAD, 11 7521 THANIKOTTAGAM PO, VEDAI, NAGAPATTINAM- 614716 K.PALANIVEL, S/O KANNIYAN, SETHINIPURAM,ROAD ST, 12 7522 VIKIRAPANDIYAM, KUDAVASAL TK, TIRUVARUR DT INDIRAGANDHI R, D/O. RAJAMANIKAM , SOUTH ST, PILLAKKURICHI PO, 13 7523 VARIYENKAVAL SENDURAI TK- ARIYALUR-621806 R.ARIVAZHAGAN, S/O RAJAMANICKAM, MELUR PO, 14 7524 KALATHUR VIA, JAYAKONDAM TK- ARIYALUR-0 PANNEERSELVAM.S, S/ O SUBRAMANIAN, PUTHUKUDI PO, 15 7525 VARIYANKAVAL VIA, UDAYARPALAYAM TK- ARIYALUR-621806 P.VELMURUGAN, S/O.P ANDURENGAN,, MULLURAKUNY VGE,, 16 7526 ADAMKURICHY PO,, ARIYALUR-0 Page 3 of 108 CANDIDATE NAME SL.NO APP.NO AND ADDRESS B.KALIYAPERUMAL, S/ O.T.S.BOORASAMY,, THANDALAI NORTH ST, 17 7527 KALLATHUR THANDALAI PO, UDAYARPALAYAM TK.- ARIYALUR L.LAKSHMIKANTHAN, S/O.M.LAKSHMANAN, SOUTH STREET,, 18 7528 THIRUKKANOOR PATTI, THANJAVUR-613303 SIVAKUMAR K.S., S/O.SEPPERUMAL.E.