Metric Variation on the Arikara Pelvis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hybrid Solution Combining Osteosynthesis and Endoprosthesis for Double Column Acetabular Fractures in the Elderly Provide More Stability with Finite Element Model
Eklem Hastalıkları ve Eklem Hastalik Cerrahisi Cerrahisi 2019;30(2):106-111 Joint Diseases and Related Surgery Original Article / Özgün Makale doi: 10.5606/ehc.2019.66592 Hybrid solution combining osteosynthesis and endoprosthesis for double column acetabular fractures in the elderly provide more stability with finite element model Yaşlılarda çift kolon asetabuler kırıklar için osteosentez ve endoprotez hibrid kombinasyonu “finite element” yöntemi ile daha çok stabilite sağlar András Kocsis, MD1, Károly Váradi, MD2, Gábor Szalai2, Tamás Kovács, MD1, Tamás Bodzay, MD, PhD1 1Jenő Manninger National Institute of Traumatology, Budapest, Hungary 2Budapest University of Technology and Economics, Faculty of Mechanical Engineering, Department of Machine and Product Design, Hungary ABSTRACT ÖZ Objectives: This study aims to compare mechanical Amaç: Bu çalışmada yaşlılarda çift kolon kırıklarında stability of osteosynthesis (plate and screw fixation) alone osteosenteze (plak ve vida tespiti) kıyasla kalça artroplastisi ile versus the same method supplemented with hip arthroplasty desteklenen aynı yöntemin (hibrid çözüm) mekanik stabilitesi (hybrid solution) for double column fractures in elderly. karşılaştırıldı. Patients and methods: Mechanical investigations were Hastalar ve yöntemler: Çift kolon kırıkları için geliştirilen performed on an advanced finite element pelvis model ileri sonlu eleman pelvis modelinde mekanik araştırmalar developed for double column fractures. The following yapıldı. İncelenen simüle edilen implant kombinasyonları simulated -

Intrapelvic Causes of Sciatica: a Systematic Review
DOI: 10.14744/scie.2020.59354 Review South. Clin. Ist. Euras. 2021;32(1):86-94 Intrapelvic Causes of Sciatica: A Systematic Review 1 1 1 1 Ahmet Kale, Betül Kuru, Gülfem Başol, Elif Cansu Gündoğdu, 1 1 2 3 Emre Mat, Gazi Yıldız, Navdar Doğuş Uzun, Taner A Usta 1Department of Gynecology and Obstetrics, University of Health Sciences, Kartal Dr. Lütfi Kırdar Training and Research Hospital, İstanbul, Turkey 2Department of Gynecology and Obstetrics, Midyat State Hospital, Mardin, Turkey 3Department of Gynecology and Obstetrics, Acıbadem University, Altunizade Hospital, İstanbul, Turkey ABSTRACT Submitted: 09.09.2020 The sciatic nerve is the nerve of the lower limb. It is derived from spinal nerves, fourth Accepted: 27.11.2020 Lumbar (L4) to third Sacral (S3). The sciatic nerve innervates the muscles of the posterior Correspondence: Ahmet Kale, thigh and additionally has sensory functions. Sciatica is the given name to the pain sourced by SBÜ Kartal Dr. Lütfi Kırdar Eğitim irritation of the sciatic nerve. Sciatica is most commonly induced by compression of a lower ve Araştırma Hastanesi, Kadın lumbar nerve root (L4, L5, or S1). Various intrapelvic pathologies include gynecological, Hastalıkları ve Doğum Kliniği, İstanbul, Turkey vascular, traumatic, inflammatory, and tumoral disorders that may cause sciatica. Intrapelvic E-mail: [email protected] pathologies that mimic disc herniation are quite always ignored. Surgical approach and a functional exploration by laparoscopy or robotic surgery have significantly increased the intrapelvic pathology’s awareness, resulting in sciatica. After a detailed assessment of the patient, which causes intrapelvic pathologies, deciding whether surgical or medical therapy is needed, notable results in sciatic pain remission can be done. -

The Pelvis Structure the Pelvic Region Is the Lower Part of the Trunk
The pelvis Structure The pelvic region is the lower part of the trunk, between the abdomen and the thighs. It includes several structures: the bony pelvis (or pelvic skeleton) is the skeleton embedded in the pelvic region of the trunk, subdivided into: the pelvic girdle (i.e., the two hip bones, which are part of the appendicular skeleton), which connects the spine to the lower limbs, and the pelvic region of the spine (i.e., sacrum, and coccyx, which are part of the axial skeleton) the pelvic cavity, is defined as the whole space enclosed by the pelvic skeleton, subdivided into: the greater (or false) pelvis, above the pelvic brim , the lesser (or true) pelvis, below the pelvic brim delimited inferiorly by the pelvic floor(or pelvic diaphragm), which is composed of muscle fibers of the levator ani, the coccygeus muscle, and associated connective tissue which span the area underneath the pelvis. Pelvic floor separate the pelvic cavity above from the perineum below. The pelvic skeleton is formed posteriorly (in the area of the back), by the sacrum and the coccyx and laterally and anteriorly (forward and to the sides), by a pair of hip bones. Each hip bone consists of 3 sections, ilium, ischium, and pubis. During childhood, these sections are separate bones, joined by the triradiate hyaline cartilage. They join each other in a Y-shaped portion of cartilage in the acetabulum. By the end of puberty the three bones will have fused together, and by the age of 25 they will have ossified. The two hip bones join each other at the pubic symphysis. -

HUMAN PELVIS HEIGHT IS ASSOCIATED with OTHER PELVIS MEASUREMENTS of OBSTETRIC VALUE Munabi Ian G
ORIGINAL COMMUNICATION Anatomy Journal of Africa. 2015. Vol 4 (1): 457 – 465 HUMAN PELVIS HEIGHT IS ASSOCIATED WITH OTHER PELVIS MEASUREMENTS OF OBSTETRIC VALUE Munabi Ian G. *, Mirembe Florence, Luboga Sam A *Corresponding author; Munabi Ian G, Department of Anatomy, School of Biomedical Sciences, Makerere University College of Health Sciences, P.O. Box 7072 Kampala Uganda East Africa Telephone: +256772485474 Email: [email protected] ABSTRACT In low resource settings, perinatal death remains a major challenge, yet some of the key anthropometric measures used for screening have been found to be inappropriate. These calls for additional anatomically related measurements to act as a basis for the design of: easy-to-use, low technology accurate tools to enhance obstetric care quality in these settings. This study set out to determine the associations between the various pelvis anthropometric measurements of obstetric importance with pelvis height. The study made use of 30 complete rearticulated Adult pelvic bonesets of known sex. The some of the thirteen measurements made on each boneset included: Pelvis height, Sacral Anterior Orientation (SAO), pubic bone length, total pelvis height and inlet medial-lateral diameter. All measurements were taken thrice and the average used for comparisons with pelvis height. The non-parametric Mann-Whitney test and multilevel regression analysis test to control for gender was used. Pelvis height had significant associations with SAO (-0.36, P<0.01), pubic bone length (0.41, P<0.01), total pelvis height (0.21, P=0.04) and inlet medial-lateral diameter (0.46, P=0.02). Additional significant associations were observed with the diameters of the mid and outlet diameters of the birth canal. -

The False Pelvis Lies Superior to the True Pelvis and Linea Terminalis. It Is a Larger and Wider Basin That Supports the Enlarge
A. Obstetric Pelvis 1. What is the job of the false pelvis? The false pelvis lies superior to the true pelvis and linea terminalis. It is a larger and wider basin that supports the enlarged uterus during pregnancy, as well as the intestines. 2. Name the 3 parts of the true pelvis. The three parts of the true pelvis are the inlet, the pelvic cavity, and the pelvic outlet. 3. What are the 4 classifications of the pelvis? The four classifications of the pelvis are the gynecoid, the android, the anthropoid, and the platypelloid. 4. Concerning the above types of pelvises, what effect does each have on: labor, engagement of the fetal head, and overall birth prognosis? The gynecoid pelvis during labor reduces perineal tears during labor, and contributes to spontaneous delivery. The uterus functions well, reducing in size when it’s supposed to. The fetus also successfully rotates throughout the birth canal early, and completely. The fetal head will engage in the transverse or oblique diameter, meaning the back of the head will face the lateral or diagonal sides of the pelvis such as the pectineal or arcuate line. With the head engaged in these starting positions, the fetus will also be in a slight asynclitism, meaning the fetus’ head will be tipped towards one shoulder, with its body and head not going in a completely straight line downwards. The fetus has good flexion, with his chin tucked in his chest and is in the common occiput anterior position, where the back of his head is facing down and toward the pubic symphysis. -

Comparison of Different Fixation Methods of Bicolumnar Acetabular Fractures
Eklem Hastalıkları ve Eklem Hastalik Cerrahisi Cerrahisi 2018;29(1):2-7 Joint Diseases and Related Surgery Original Article / Özgün Makale doi: 10.5606/ehc.2018.59268 Comparison of different fixation methods of bicolumnar acetabular fractures Çift kolonlu asetabulum kırıklarının farklı tespit yöntemlerinin karşılaştırılması Tamás Bodzay, MD, PhD,1 Gergely Sztrinkai, MD,1 András Kocsis, MD,1 Báliinstnt Kozma,3 Tamás Gál, MD,2 Károly Váradi, PhD, D.Sc.3 1Trauma Centre, Péterfy Hospital, Budapest, Hungary 2 Department of Trauma, Semmelweis University, Budapest, Hungary 3Faculty of Mechanical Engineering, Institute of Machine Design, Budapest, Hungary ABSTRACT ÖZ Objectives: This study aims to investigate if the stabilization Amaç: Bu çalışmada iliyak kanat kırıklarının of iliac wing fractures influences the stability of the acetabular stabilizasyonunun asetabüler osteosentezin stabilitesini osteosynthesis, if surgical fixation is the choice of treatment, etkileyip etkilemediği, cerrahi tespitin tercih edilen tedavi olup and which technique to be used. olmadığı ve kullanılacak tekniğin hangisi olduğu araştırıldı. Materials and methods: In the study, measurements were Gereç ve yöntemler: Çalışmada ölçümler iyileştirilmiş bir performed with an improved finite element model. Tension sonlu eleman modeli ile yapıldı. Çift kolonlu asetabulum and displacement values were measured in bicolumnar kırıklarında gerginlik ve displasman değerleri aşağıdaki acetabular fractures in the following cases: combination of olgularda ölçüldü: Linea terminalis boyunca kraniyal ve cranial and medial plate fixation through the linea terminalis, medial plak tespiti kombinasyonu veya kraniyal plak ve or combination of cranial plate and quadrilateral surface dörtgen yüzeyli plak kombinasyonu. İliyak kanat kırığı plates. The iliac wing fracture was either not fixed, or fixed ya tespit edilmedi ya da vidalarla veya bir plak ile tespit with screws or with a plate. -
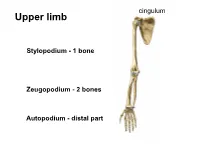
Alekzs02.Pdf
cingulum Upper limb • S Stylopodium - 1 bone Zeugopodium - 2 bones Autopodium - distal part Shoulder (pectoral) girdle 2 • Klíček 4 1 • Lopatka 3 • PH 5 1 StC 2 1 2 AC 3 GH 4 3 4 SuA 5 TSc junction 5 1 Articulatio sternoclavicularis 2 Articulatio acromioclavicularis 9 2 discus 1 4 6a 6b 3 5 10 7 8 3- lig. interclaviculare 4-lig. sternoclaviculare 5-lig costoclaviculare 6-lig. coracoclaviculare a) lig. trapezoideum b) lig. conoideum 7-lig. coracoacromiale (fornix humeri) 8-lig. coracohumerale 9- lig. acromioclaviculare 10 – lig. transversum scapulae Articulatio acromioclavicularis C-C C-A C-H Scapula movements (Movements in thoracoscapular junction) with movements in StC Th3 and AC joint retraction protraction Th7 elevation depression rotation midposition – palm on the neck Ligamentum coracoclaviculare the strongest stabilizer of the AC joint. conoid trapezoid Long head BiBr Labrum glenoidale Glenoid lip Long headTriBr Superior aspect of the acromioclavicular joint 1 P L 1 M Lig. transversum Fornix humeri scapulae superius A X-ray https://www.auntminnie.com/index.aspx?sec=olce&sub=cotx&pag=cpages&ce_id=11221&pno=1 X-ray 1 2 3 7 5 6 4 1- extremitas acromialis 2-acromion 3-greater turebcle 4-lesser tubercle 5-caput humeri 6-cavitas glenoidalis 7-coracoid process Synovial joint description • 1) type and shape of joint General schema of synovial joint • 2) articular surfaces • 3) joint capsule attachment • 4) joint capsule reinforcement – ligaments, muscles • 5) range of movements • 6) midposition • 7) scheme 1-ball (head) 2- collateral ligament 3-fibrous capsule 4-synovial capsule 5- articular cartilage (hyaline) 6-articular lip (labrum 7-socket (fossa) 8- muscle with tendon 9-ligament 10-synovial bursa communicating with the joint cavity 11-menisc 12-synovial bursa (subtendinea) Art. -

The Female Pelvis in Point of Obstetrics from Considerations of Obstetrics the Female Pelvis Is Divided Into Two Parts: Large and Small Pelvis
Women’s pelvis. Fetus as object of births. 1. Relevance of the topic: The topic is major in obstetrics. Without it, further study of obstetric discipline, mastering many topics of physiological and pathological obstetrics is impossible. Knowledge of the anatomy of the pelvis, the ability to measure its size allows you to assess the correspondence between the size of the birth canal and the fetal head, ie to predict the course and outcome of childbirth. 2. Specific goals: -To know the structure of a woman's pelvis, the boundaries and dimensions of the pelvic planes - To classify the main external and additional dimensions of the pelvis - To measure the external dimensions of the pelvis - To set the size of the true conjugate - To know the structure of the fetal skull, the location of sutures, temples - To determine the size of the head and torso of the fetus - To interpret the signs of fetal maturity. 3. The basic level of expertise, skills, abilities, required for learning the topic (interdisciplinary integration ) Names of previous disciplines Skills acquired 1. Normal anatomy Knowledge of female anatomy, genitals, structure of a woman's pelvis, types of bone connections, musculo-fascial systems, blood supply to the female genitals. 2. Pediatrics Knowledge of the structure of the newborn, placement of tendrils, age norms of their closing 4. Tasks for independent work during preparation to the lesson and in the lesson. 4.1. A list of basic terms, parameters, characteristics that a student must learn during preparation to the lesson. Term Definition 1. Distantia spinarum The distance between the anterior spines of iliac bones - 25 cm 2. -

Bones of the Hindlimb
BONES OF THE HINDLIMB Andrea Heinzlmann University of Veterinary Medicine Budapest Department of Anatomy and Histology 1st Oktober 2019 BONES OF THE HINDLIMB COMPOSED OF: 1. PELVIC GIRDLE (CINGULUM MEMBRI PELVINI) 2. THIGH 3. LEG (CRUS) 4. FOOT (PES) BONES OF THE PELVIC LIMB (OSSA MEMBRI PELVINI) PELVIC GIRDLE (CINGULUM MEMBRI PELVINI): - connection between the pelvic limb and the trunk consists of: 1. two HIP BONES (OSSA COXARUM) Hip bones of a pig, dorsal aspect Hip bones of a pig, ventral aspect PELVIC GIRDLE (CINGULUM MEMBRI PELVINI) HIP BONE (OS COXAE): - in young animals each hip bone comprises three bones: 1. ILIUM (OS ILII) – craniodorsal 2. PUBIS (OS PUBIS) – cranioventral 3. ISHIUM (OS ISCHI) – caudoventral - all three bones united by a synchondrosis - the synchondrosis ossifies later in life Hip bones of an ox, left lateral aspect Hip bones of an ox, ventrocranial aspect PELVIC GIRDLE (CINGULUM MEMBRI PELVINI) HIP BONE (OS COXAE): ACETABULUM: - ilium, pubis and ischium meet at the acetabulum Left acetabulum of a horse, lateral aspect Hip bones of a dog, right lateral aspect Left acetabulum of an ox, lateral aspect PELVIC GIRDLE (CINGULUM MEMBRI PELVINI) HIP BONE (OS COXAE): SYMPHYSIS PELVINA: - the two hip bones united ventrally at the symphysis pelvina by a fibrocartilaginous joint ossified with advancing age - in females the fibrocartilage of the symphysis becomes loosened during pregnancy by action of hormones Hip bones of a horse, ventrocranial aspect Hip bones of an ox, ventrocranial aspect PELVIC GIRDLE (CINGULUM MEMBRI PELVINI) HIP BONE (OS COXAE): SYMPHYSIS PELVINA: - in females the fibrocartilage of the symphysis becomes loosened during pregnancy by action of hormones http://pchorse.se/index.php/en/articles/topic-of-the-month/topics-topics/4395-mars2017-eng PELVIC GIRDLE (CINGULUM MEMBRI PELVINI) HIP BONE (OS COXAE): SYMPHYSIS PELVINA divided into: 1. -

Pelvic Cavity
Pelvic Cavity http://www.smbs.buffalo.edu/ana/newpage45.htm [ Home ] [ Up ] [ Anterior Body Wall ] [ Embryonic Body Cavity ] [ Lunglecture ] [ Mediastinum ] [ Heart ] [ Autonomic NS ] [ Rotation of the Gut/Abdomen ] [ Abdominal-Peritoneal Cavities ] [ Glands ,Lymphoid Organs and Blood Supply ] [ Pelvic Cavity ] [ Posterior body wall ] [ Perineum ] [ Home ] [ Up ] [ Anterior Body Wall ] [ Embryonic Body Cavity ] [ Lunglecture ] [ Mediastinum ] [ Heart ] [ Autonomic NS ] [ Rotation of the Gut/Abdomen ] [ Abdominal-Peritoneal Cavities ] [ Glands ,Lymphoid Organs and Blood Supply ] [ Pelvic Cavity ] [ Posterior body wall ] [ Perineum ] Fall 1999 Moore, pp 332-388 Lecture 19 Dr. C. Dlugos PELVIC CAVITY AND ORGANS Overview: An understanding of the pelvis and the organs contained within it is essential in several medical disciplines such as gynecology, obstetrics, urology and gastroenterology. The functions of the pelvis include protection of the viscera, composition of the girdle by which the lower limb is attached to the axial skeleton, and an attachment site for the external genitalia. This lecture should provide the student with basic information concerning the pelvis, differences in the pelvis within the sexes, and a general knowledge of the anatomy of the pelvic organs in males and females. Objectives 1. to learn the anatomy of the bony pelvis and what regions comprise the false and the true pelvis 2. to be aware of sexual differences in the pelvis 3. to understand the anatomy of the pelvic diaphragm in the male and the female 4. to understand the anatomy of the pelvic organs in the male and the female Pelvis:(Latin for basin), region of transition where the trunk and the lower limbs meet, inferior portion of the trunk, contains pelvic cavity, the inferior portion of the abdominopelvic cavity, below the pelvic brim. -

General Principles the Acetabular Index and Center-Edge Angles
Troelson E-Appendix Page 1 of 14 E-Appendix General Principles The acetabular index and center-edge angles following reorientation should correspond as closely as possible to the normal anatomy (acetabular index angle, 0° to10°; center-edge angle, 30° to 40°). It is of equal importance that the surgeon obtain appropriate anteversion of the acetabulum. Assessment of the range of motion and joint stability at the end of the procedure will help the surgeon to evaluate the change in hip joint mechanics. This description of the minimally invasive approach will give the reader an understanding of soft- tissue mobilization, instrument handling, and the performance of the osteotomies. Understanding of the anatomy and utilization of fluoroscopy during surgery are the keys to a safe, minimally invasive periacetabular osteotomy. Pubic Osteotomy Subperiosteal access to the superior ramus of the pubic bone is gained with use of a periosteal elevator. It is important that the pubic osteotomy be performed medially on the superior ramus since the bone otherwise is too thick, making the osteotomy and mobilization difficult or impossible. A curved blunt retractor is placed in the obturator fossa behind the superior ramus of the pubic bone. It is important that this retractor be placed subperiosteally to protect the obturator artery and nerve. A splined retractor is then placed anterior and medial to the site of the osteotomy in order to retract the iliopsoas muscle medially and protect the iliac artery and vein and the femoral nerve (Fig. E1). The superior ramus is then osteotomized under direct visualization with use of a slightly curved osteotome. -
HS 2017 MED-X620 Intro to Pelvis & Perineum; Anal Triangle
K.E. Byrd X620 2017 10-16-2017 HS 2017 MED-X620 Intro to Pelvis & Perineum; Anal Triangle Kenneth E. Byrd, PhD [email protected] 274-3355 HS 2017 MED-X620 IUSOM Osteology Bony Pelvis – hip bones, sacrum and coccyx •Protect Pelvic Viscera •Support Body Weight. Superior •Attachments: muscles for Abdomen & LE Hip Bones - Os Coxae -Innominate Bone • Start as 3 bones separated by hyaline ilium cartilage called the Tri-radiate cartilage; located at the acetabulum. • They complete fusion at ~12 years for Posterior Anterior girls and ~14 years for boys. pubis ischium Lateral View of immature Ox Coxa Inferior P&P_1_Intro Pelvis-Perineum; Anal Triangle 1 of 17 K.E. Byrd X620 2017 10-16-2017 Sagittal Section Abdominal Pelvic Cavity Pelvic Inlet (pelvic brim) = skeletal (striated) muscles, GSE innervated…… aka Pelvic Diaphragm!!!!!!! Osteology Pelvic Girdle • 2 hip bones • sacrum • coccyx • sacroiliac joints • pubic symphysis (fibrocartilage) Pelvic Inlet (brim) Linea Terminalis (obstetrical term) - from pubic symphysis (anterior) to sacroiliac (SI) joint and the sacral promontory (SP) (posterior) Pectineal line completes the pelvic inlet Pubic tubercle P&P_1_Intro Pelvis-Perineum; Anal Triangle 2 of 17 K.E. Byrd X620 2017 10-16-2017 Pelvic Fractures & Common Causes Pelvic Fractures • High‐Energy Trauma • Congenital Bone Development • Osteoporosis • Avulsion – ischial tuberosity (hamstring) • Acetabulum • Types: Stable vs. Unstable Unstable fracture. In this type of fracture, there are usually two or more breaks in the pelvic ring and the ends of the broken bones do not line up correctly (displacement). Stable fracture. In this type of This type of fracture is more likely to occur due to fracture, there is often only one a high‐energy event.