Host Range Of, and Plant Reaction To, Subanguina Picridis 1 A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COST EFFECTIVE PRODUCTION of SPECIALTY CUT FLOWERS By
COST EFFECTIVE PRODUCTION OF SPECIALTY CUT FLOWERS By TODD JASON CAVINS Bachelor of Science Southwestern Oklahoma State University Weatherford, Oklahoma 1997 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE December, 1999 COST EFFECTIVE PRODUCTION OF SPECIALTY CUT FLOWERS Thesis Approved: ' 1 Thesis Advisor .. ;.; ,, ( Dean of the Graduate College 11 ACKNOWLEDGEMENTS The purpose of this study was to improve production methods of various specialty cut flower species. Improving production methods allows growers to reduce cost, improve plant quality and earn higher profits. This study involved three research areas of specialty cut flowers. Partial funding was provided by a S.A.R.E. grant and Bear Creek Farm, Stillwater, OK. I would like to thank my principle advisor Dr. John Dole for his encouragement, support, honesty and perseverance. I would like to thank Dr. Janet Cole and Dr. Jim Ownby for serving on my thesis committee. Dr. Cole offered valuable insight and direction towards the research. Dr. Ownby contributed with his wealth of knowledge in plant physiology. A special thanks goes to Vicki Stamback and the gang at Bear Creek Farm. Vicki's experience as a specialty cut flower grower allowed me to gain personal knowledge of the cut flower industry that would not have taken place without her. Vicki's efforts and cooperation greatly improved this study. I want to thank Randall Smith and Leah Aufill for their assistance and plant care. Tim Hooper also contributed by offering his experiences from the floriculture industry and providing stress relieving lunch breaks. -

Heart of Uwchlan Pollinator Garden Plant Suggestions – Perennials 2020 Page 1
Pollinator Garden Plant Suggestions - Perennials Heart of Uwchlan Project Tips for Planting a Pollinator Garden • Assess your location. Is it dry? Often wet? Is soil clay or loamy? How much sun or shade? Select plants appropriate to the conditions: “Right plant in the right place.” • Plant so you have blooms in every season. Don’t forget late summer/autumn bloomers; migrating butterflies need that late season pollen and nectar. • Plant for a variety of flower color and shape. That’s prettier for you, but it also appeals to a variety of pollinators. Some bees and butterflies prefer specific plants. • Plant in groups of at least three . easier for pollinators to find and browse. • Don’t forget the birds. Plant tubular flowers for hummingbirds, bushes with berries for birds (see related Plant List for Shrubs). • Finally, do minimal cleanup in the fall. Leave the leaves, dead stems and flower heads. Beneficial insects like miner bees lay eggs in hollow stems, finches will eat the echinacea seeds. Many butterflies and moths overwinter as pupae in dead leaves. Spring Blooming Golden-ragwort (Packera aurea) – mid to late Spring – Damp location, shade Grows freely and naturalizes into large colonies. Yellow flower heads, blooms for over 3 weeks in mide- to late spring. Dense ground cover. Prefers partial sun, medium shade. Prefers moist, swampy conditions. Cut back bloom stalks after flowering. Golden Alexander (Zizia aurea) – blooms May-June – prefers wet habitats but will tolerate dry Attractive bright yellow flower which occurs from May – June, looks like dill in shape. An excellent addition to a wildflower garden because it provides accessible nectar to many beneficial insects with short mouthparts during the spring and early summer when such flowers are relatively uncommon. -

Quantifying Habitat and Landscape Effects on Composition and Structure of Plant-Pollinator Networks in the US Northern Great
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.12.431025; this version posted February 13, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Quantifying habitat and landscape effects on composition and structure 2 of plant-pollinator networks in the US Northern Great Plains 3 4 5 Isabela B. Vilella-Arnizaut, Corresponding Author1 6 Department of Biology and Microbiology, South Dakota State University, 1390 College 7 10 Ave, Brookings, SD 57007 8 Charles Fenster2 9 Director Oak Lake Field Station, South Dakota State University, Brookings, South 10 Dakota, USA 11 Henning Nottebrock3 12 Department of Biology and Microbiology, South Dakota State University, Brookings, 13 South Dakota, USA 14 15 16 [email protected] 17 [email protected] 18 [email protected] 19 3Current address: Plant Ecology, Bayreuth Center of Ecology and Environmental Research (BayCEER), University 20 of Bayreuth, Universitätsstr. 30, Bayreuth, Germany bioRxiv preprint doi: https://doi.org/10.1101/2021.02.12.431025; this version posted February 13, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 21 Abstract 22 Community structure contributes to ecosystem persistence and stability. To understand the 23 mechanisms underlying pollination and community stability of natural areas in a human 24 influenced landscape, a better understanding of the interaction patterns between plants and 25 pollinators in disturbed landscapes is needed. -

Vascular Plants and a Brief History of the Kiowa and Rita Blanca National Grasslands
United States Department of Agriculture Vascular Plants and a Brief Forest Service Rocky Mountain History of the Kiowa and Rita Research Station General Technical Report Blanca National Grasslands RMRS-GTR-233 December 2009 Donald L. Hazlett, Michael H. Schiebout, and Paulette L. Ford Hazlett, Donald L.; Schiebout, Michael H.; and Ford, Paulette L. 2009. Vascular plants and a brief history of the Kiowa and Rita Blanca National Grasslands. Gen. Tech. Rep. RMRS- GTR-233. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 44 p. Abstract Administered by the USDA Forest Service, the Kiowa and Rita Blanca National Grasslands occupy 230,000 acres of public land extending from northeastern New Mexico into the panhandles of Oklahoma and Texas. A mosaic of topographic features including canyons, plateaus, rolling grasslands and outcrops supports a diverse flora. Eight hundred twenty six (826) species of vascular plant species representing 81 plant families are known to occur on or near these public lands. This report includes a history of the area; ethnobotanical information; an introductory overview of the area including its climate, geology, vegetation, habitats, fauna, and ecological history; and a plant survey and information about the rare, poisonous, and exotic species from the area. A vascular plant checklist of 816 vascular plant taxa in the appendix includes scientific and common names, habitat types, and general distribution data for each species. This list is based on extensive plant collections and available herbarium collections. Authors Donald L. Hazlett is an ethnobotanist, Director of New World Plants and People consulting, and a research associate at the Denver Botanic Gardens, Denver, CO. -
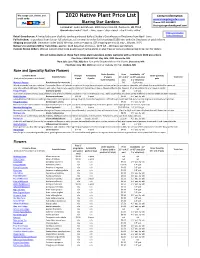
2020 Native Plant Price List
We accept cash, checks, and 2020 Native Plant Price List Contact information: credit cards: www.blazingstargardens.com Blazing Star Gardens Phone: 507-402-8337 [email protected] Located at: Souba Greenhouse, 4003 Crane Creek Rd, Owatonna, MN 55060 Greenhouse hours*: April - June, open 7 days a week - check hours online* *Hours and Location Retail Greenhouse: A limited selection of plants can be purchased daily at Souba's Greenhouse in Owatonna from April - June. Souba Greenhouse Full selection: To purchase from for our full selection, call or email to order for free pickup ($100 min. order) in Owatonna or paid delivery. Shipping across USA: (minimum order $100) We ship, costs are approx. $25 shipping per tray (1 tray = 18 pots, 3.5") Delivery to southern MN or Twin Cities: approx. $125 (less than 15 trays) - $275 (15 - 100 trays) per delivery Custom Grown Orders: We can custom grow large quantities of native plants in plug trays or pots at a discounted price. Call for details. We sell native plants at these Twin Cities plant sales (pre-orders welcome with a minimum $100 pre-order): Plant Sale: CANCELLED Sat. May 16th, 2020 Burnsville, MN Plant Sale: June TBD, 2020 9am-1pm at the Shepherd of the Hills Church, Shoreview, MN Plant Sale: June TBD, 2020 9am-1pm at Oakdale City Hall, Oakdale, MN Rare and Specialty Native Flowers Order Quantity Price Availability 3.5" Common Name Price per Availability Order Quantity Scientific Name 2" plants 3.5" or 4.5" or 4.5" pots (1 or Total Cost (click plants for pictures and details) 6-pack 6-packs pots (sold in 6-packs) pots 2 yr old plants) Monarch Factory Porch planter for monarchs - - $49 6 gallon pot Watch monarchs from your window! Our popular Monarch Factories are planted in large decorative pots and are perfect for porches or sidewalks, with plants that provide food for monarch caterpillars (Marsh Milkweed flowers), and nectar that attracts swarms of monarch butterflies in August (Meadow Blazing Star flowers). -

Color and Common Name Prairie Plant List
Native or Likely Color Common Name Scientific Name Family Introduced to See Orange N Butterflyweed Asclepias tuberosa var. interior Asclepiadaceae X Orange N Michigan Lily Lilium michiganense Liliaceae Pink I Alsike Clover Trifolium hybridum Fabaceae X Pink N American Vetch Vicia americana Fabaceae X Pink I Bouncing Bet Saponaria officinalis Caryophyllaceae X Pink I Bull Thistle Cirsium vulgare Asteraceae X Pink I Canada Thistle Cirsium arvense Asteraceae X Pink N Canada Tick Trefoil Desmodium canadense Fabaceae X Pink I Catnip Nepeta cataria Lamiaceae X Pink N Common Milkweed Asclepias syriaca Asclepiadaceae X Pink I Crownvetch Coronilla varia Fabaceae X Pink N Dotted Blazing Star Liatris punctata var. punctata Asteraceae X Pink N Great Blazing Star Liatris pycnostachya var. pycnostachya Asteraceae X Pink N Heart-Leaved Four O'Clock Mirabilis nyctaginea Nyctagenaceae X Pink N Hill's Thistle Cirsium pumilum var. hillii Asteraceae Pink N Northern Plains Blazing Star Liatris ligulistylis Asteraceae Pink N Obedient Plant Physostegia virginiana var. virginiana Lamiaceae X Pink N Prairie Phlox Phlox pilosa var. fulgida Polemoniaceae X Pink N Prairie Rose Rosa arkansana Rosaceae X Pink N Prairie Smoke Geum triflorum Rosaceae X Pink N Prairie Wild Onion Allium stellatum Liliaceae Pink I Rabbit's Foot Clover Trifolium arvense Fabaceae X Pink I Red Clover Trifolium pratense Fabaceae X Pink N Rough Blazing Star Liatris aspera Asteraceae X Pink I Spotted Knapweed Centaurea stoebe subsp. micranthos Asteraceae X Pink N Spreading Dogbane Apocynum androsaemifolium Apocynaceae X Pink N Swamp Milkweed Asclepias incarnata var. incarnata Asclepiadaceae X Pink I Tartarian Honeysuckle Lonicera tatarica Caprifoliaceae X Pink N Wild Bergamot Monarda fistulosa Lamiaceae X Pink N Wild Garlic Allium canadense var. -

Genetic Diversity and Evolution in Lactuca L. (Asteraceae)
Genetic diversity and evolution in Lactuca L. (Asteraceae) from phylogeny to molecular breeding Zhen Wei Thesis committee Promotor Prof. Dr M.E. Schranz Professor of Biosystematics Wageningen University Other members Prof. Dr P.C. Struik, Wageningen University Dr N. Kilian, Free University of Berlin, Germany Dr R. van Treuren, Wageningen University Dr M.J.W. Jeuken, Wageningen University This research was conducted under the auspices of the Graduate School of Experimental Plant Sciences. Genetic diversity and evolution in Lactuca L. (Asteraceae) from phylogeny to molecular breeding Zhen Wei Thesis submitted in fulfilment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. Dr A.P.J. Mol, in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Monday 25 January 2016 at 1.30 p.m. in the Aula. Zhen Wei Genetic diversity and evolution in Lactuca L. (Asteraceae) - from phylogeny to molecular breeding, 210 pages. PhD thesis, Wageningen University, Wageningen, NL (2016) With references, with summary in Dutch and English ISBN 978-94-6257-614-8 Contents Chapter 1 General introduction 7 Chapter 2 Phylogenetic relationships within Lactuca L. (Asteraceae), including African species, based on chloroplast DNA sequence comparisons* 31 Chapter 3 Phylogenetic analysis of Lactuca L. and closely related genera (Asteraceae), using complete chloroplast genomes and nuclear rDNA sequences 99 Chapter 4 A mixed model QTL analysis for salt tolerance in -

2021 Native Plant Price List
We accept cash, checks, and 2021 Native Plant Price List Contact information: credit cards: www.blazingstargardens.com Blazing Star Gardens Phone: 507-402-8337 Located at: Souba Greenhouse, 4003 Crane Creek Rd, Owatonna, MN 55060 [email protected] Greenhouse hours*: April - June, open 7 days a week - check hours online* Easy Online Ordering at: www.blazingstargardens.com Pre-Ordering: The best way to order or pre-order is through our easy-to-use Online Store at www.blazingstargardens.com *Hours and Location Retail Greenhouse: A limited selection of plants can be purchased daily at Souba's Greenhouse in Owatonna from April - June. Souba Greenhouse Shipping across USA: We ship to most Midwest and East Coast states (see our Online Store to order and for more details) Twin Cities Native Plant Sales: We sell native plants at these Twin Cities plant sales: Plant Sale (pre-orders only): Burnsville, MN - May 15th, 2020 - 9am- 12pm at the parking lot across from City Hall, 100 Civic Center Parkway Plant Sale: Shoreview, MN - June 5th, 2020 9am-1pm at the Shepherd of the Hills Church, Shoreview, MN Plant Sale: Oakdale, MN - June 12th, 2020 9am-1pm at Oakdale City Hall, Oakdale, MN Rare and Specialty Native Flowers Availability Price Common Name Price per 6- Order Quantity Availability Order Quantity Scientific Name 6-packs or 3.5" or 4.5" Total Cost (click plants for pictures and details) pack or tray 6-packs or trays 3.5" or 4.5" pots pots trays pots Monarch Factory Porch planter for monarchs - - $49 6 gallon pot Watch monarchs from your window! Our popular Monarch Factories are planted in large decorative pots and are perfect for porches or sidewalks, with plants that provide food for monarch caterpillars (Marsh Milkweed flowers), and nectar that attracts swarms of monarch butterflies in August (Meadow Blazing Star flowers). -

"Shade Affects Yield and Stem Length of Field-Grown Cut-Flower Species"
HORTSCIENCE 26(9):1174-1176. 1991. Halevy, 1975), geranium (Armitage and Wetzstein, 1984; Craig and Walker, 1963), Shade Affects Yield and Stem Length and carnations (Bunt, 1973; Holley, 1959) is reduced or delayed as irradiance is re- duced. Providing shade is an effective means of Field-grown Cut-flower Species of reducing irradiance, but low irradiance has A.M. Armitage also been shown to increase internode elon- gation (Armitage et al., 1990). Department of Horticulture, University of Georgia, Athens, GA 30602 The objective of this research was to de- Additional index words. Centaurea americana, basket flower, Eryngium planum, sea termine the effects of shade on yield (flowers holly, Echinops ritro, globe thistle, Anemone coronaria, poppy anemone, Zantedeschia, per plant) and stem length of annual (Cen- calla lily, irradiance, harvest duration taurea), perennial (Echinops, Eryngium), and bulbous (Anemone, Zantedeschia) species Abstract. Various field-grown specialty cut-flower species were subjected to full sun grown in climatic zone 7b (U.S. Dept. of or 55% or 67% shade treatments for 2 to 3 years. Plants grown in shade had longer Agriculture, 1990). The influence of shade flower stems than those grown in ambient irradiance; however, yield (flower stems per on spathe width of Zantedeschia flowers was plant) was species-dependent. Yield of Centaurea americana Nutt. ‘Jolly Joker’, an also determined. annual speices, and Eryngium planum L., a perennial, declined linearly with each Raised beds, » 2 m wide and 15 m long, reduction in irradiance. However, yield of Echinops ritro L. ‘Taplow Blue’, a perennial were constructed in 1985 near Athens, Ga., species, was higher in 55% shade than in ambient irradiance. -

Pollinator Visitation Frequency Associated with Native and Non-Native Plants in a Mid-Atlantic Piedmont (USA) Urban Garden
Virginia Journal of Science Note: This manuscript has been accepted for publication and is Volume 70, Issue 1 & 2 online ahead of print. It will undergo copyediting, typesetting, and Spring & Summer 2019 doi: review of the resulting proof before it is published in its final form. Pollinator Visitation Frequency Associated with Native and Non-native Plants in a Mid-Atlantic Piedmont (USA) Urban Garden Nicholas J. Ruppel1, Saunders M. Riley2, Ellis D. Mumford3, Barbara L. Swedo4 Department of Biology, Randolph-Macon College, Ashland, VA 23005 1 [email protected] , 2 [email protected] , 3 [email protected] , 4 [email protected] ABSTRACT The recent focus on the importance of native plants and their pollinators has highlighted the critical role of local species in their natural environment. As urban encroachment, climate change, and invasive species continues to threaten native habitats, it is increasingly important to promote the use of local green spaces as refugia for native plants and their pollinators. The aim of this project, therefore, was to identify and assess the visitation frequency of insect pollinators associated with an urban setting within the Piedmont region of Virginia, and compare their association with native versus closely-related but non-native summer- flowering plants. Several modes of insect examination were used to assess these metrics in the Brian Wesley Moores Native Plant Garden on the campus of Randolph-Macon College. We observed an overall preference for the native species on a total of four native:non-native pair comparisons, including a higher number of total insect visitors and a more diverse assortment of pollinator types. -

Application of Internal Transcribed Spacer of Nuclear Ribosomal Dna for Identification of Echinops Mandavillei Kit Tan Fahad M
Bangladesh J. Plant Taxon. 21(1): 33-42, 2014 (June) © 2014 Bangladesh Association of Plant Taxonomists APPLICATION OF INTERNAL TRANSCRIBED SPACER OF NUCLEAR RIBOSOMAL DNA FOR IDENTIFICATION OF ECHINOPS MANDAVILLEI KIT TAN 1 2 3 FAHAD M.A. AL-HEMAID, M. AJMAL ALI , JOONGKU LEE , GÁBOR GYULAI 4 AND ARUN K. PANDEY Department of Botany and Microbiology, College of Science, King Saud University, Riyadh 11451, Saudi Arabia Keywords: Echinops mandavillei; Asteraceae; ITS; nrDNA; Endemic; Saudi Arabia. Abstract The present study explored the use of internal transcribed spacers (ITS) sequences (ITS1-5.8S-ITS2) of nuclear ribosomal DNA (nrDNA) for identification of Echinops mandavillei Kit Tan, an endemic species to Saudi Arabia. The sequence similarity search using Basic Local Alignment Search Tool (BLAST) and phylogenetic analyses of the ITS sequence of E. mandavillei Kit Tan showed high level of sequence similarity (98%) with E. glaberrimus DC. (section Ritropsis). The novel primary sequence and the secondary structure of ITS2 of E. mandavillei could have a potential use for molecular genotyping. Introduction The genus Echinops L. belonging to the subtribe Echinopsinae of Cynareae, of the family Asteraceae comprise about 120 species (Vidović, 2011), and distributed in tropical Africa, the Mediterranean basin, temperate regions of Eurasia, Central Asia, Mongolia and North-eastern China, with the maximum number of species occurring in the Caucasus and the Middle East (Susanna and Garcia-Jacas, 2007). The genus received considerable interest for establishing natural groups with infrageneric classification (Sánchez-Jiménez et al., 2010). Morphological characters, like the pappus, which is a key taxonomic character of Cynareae, the type and density of indumentum on stems, leaf shapes and phyllaries are considered least significance in dissemination of Echinops species (Mozaffarian, 2006; Sánchez-Jiménez et al., 2010). -

Insects and Fungi Associated with Carduus Thistles (Com Positae)
t I:iiW 12.5 I:iiW 1.0 W ~ 1.0 W ~ wW .2 J wW l. W 1- W II:"" W "II ""II.i W ft ~ :: ~ ........ 1.1 ....... j 11111.1 I II f .I I ,'"'' 1.25 ""11.4 111111.6 ""'1.25 111111.4 11111 /.6 MICROCOPY RESOLUTION TEST CHART MICROCOPY RESOLUTION TEST CHART I NATIONAL BlIREAU Of STANDARDS-1963-A NATIONAL BUREAU OF STANDARDS-1963-A I~~SECTS AND FUNGI ;\SSOCIATED WITH (~ARDUUS THISTLES (COMPOSITAE) r.-::;;;:;· UNITED STATES TECHNICAL PREPARED BY • DEPARTMENT OF BULLETIN SCIENCE AND G AGRICULTURE NUMBER 1616 EDUCATION ADMINISTRATION ABSTRACT Batra, S. W. T., J. R. Coulson, P. H. Dunn, and P. E. Boldt. 1981. Insects and fungi associated with Carduus thistles (Com positae). U.S. Department of Agriculture, Technical Bulletin No. 1616, 100 pp. Six Eurasian species of Carduus thistles (Compositae: Cynareael are troublesome weeds in North America. They are attacked by about 340 species of phytophagous insects, including 71 that are oligophagous on Cynareae. Of these Eurasian insects, 39 were ex tensively tested for host specificity, and 5 of them were sufficiently damf..ghg and stenophagous to warrant their release as biological control agents in North America. They include four beetles: Altica carduorum Guerin-Meneville, repeatedly released but not estab lished; Ceutorhynchus litura (F.), established in Canada and Montana on Cirsium arvense (L.) Scop.; Rhinocyllus conicus (Froelich), widely established in the United States and Canada and beginning to reduce Carduus nutans L. populations; Trichosirocalus horridus ~Panzer), established on Carduus nutans in Virginia; and the fly Urophora stylata (F.), established on Cirsium in Canada.