Quantifying Habitat and Landscape Effects on Composition and Structure of Plant-Pollinator Networks in the US Northern Great
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diptera: Syrphidae
This is a repository copy of The relationship between morphological and behavioral mimicry in hover flies (Diptera: Syrphidae).. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/80035/ Version: Accepted Version Article: Penney, HD, Hassall, C orcid.org/0000-0002-3510-0728, Skevington, JH et al. (2 more authors) (2014) The relationship between morphological and behavioral mimicry in hover flies (Diptera: Syrphidae). The American Naturalist, 183 (2). pp. 281-289. ISSN 0003-0147 https://doi.org/10.1086/674612 Reuse Unless indicated otherwise, fulltext items are protected by copyright with all rights reserved. The copyright exception in section 29 of the Copyright, Designs and Patents Act 1988 allows the making of a single copy solely for the purpose of non-commercial research or private study within the limits of fair dealing. The publisher or other rights-holder may allow further reproduction and re-use of this version - refer to the White Rose Research Online record for this item. Where records identify the publisher as the copyright holder, users can verify any specific terms of use on the publisher’s website. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ The relationship between morphological and behavioral mimicry in hover flies (Diptera: Syrphidae)1 Heather D. Penney, Christopher Hassall, Jeffrey H. Skevington, Brent Lamborn & Thomas N. Sherratt Abstract Palatable (Batesian) mimics of unprofitable models could use behavioral mimicry to compensate for the ease with which they can be visually discriminated, or to augment an already close morphological resemblance. -

State of Colorado 2016 Wetland Plant List
5/12/16 State of Colorado 2016 Wetland Plant List Lichvar, R.W., D.L. Banks, W.N. Kirchner, and N.C. Melvin. 2016. The National Wetland Plant List: 2016 wetland ratings. Phytoneuron 2016-30: 1-17. Published 28 April 2016. ISSN 2153 733X http://wetland-plants.usace.army.mil/ Aquilegia caerulea James (Colorado Blue Columbine) Photo: William Gray List Counts: Wetland AW GP WMVC Total UPL 83 120 101 304 FACU 440 393 430 1263 FAC 333 292 355 980 FACW 342 329 333 1004 OBL 279 285 285 849 Rating 1477 1419 1504 1511 User Notes: 1) Plant species not listed are considered UPL for wetland delineation purposes. 2) A few UPL species are listed because they are rated FACU or wetter in at least one Corps Region. 3) Some state boundaries lie within two or more Corps Regions. If a species occurs in one region but not the other, its rating will be shown in one column and the other column will be BLANK. Approved for public release; distribution is unlimited. 1/22 5/12/16 Scientific Name Authorship AW GP WMVC Common Name Abies bifolia A. Murr. FACU FACU Rocky Mountain Alpine Fir Abutilon theophrasti Medik. UPL UPL FACU Velvetleaf Acalypha rhomboidea Raf. FACU FACU Common Three-Seed-Mercury Acer glabrum Torr. FAC FAC FACU Rocky Mountain Maple Acer grandidentatum Nutt. FACU FAC FACU Canyon Maple Acer negundo L. FACW FAC FAC Ash-Leaf Maple Acer platanoides L. UPL UPL FACU Norw ay Maple Acer saccharinum L. FAC FAC FAC Silver Maple Achillea millefolium L. FACU FACU FACU Common Yarrow Achillea ptarmica L. -

Heart of Uwchlan Pollinator Garden Plant Suggestions – Perennials 2020 Page 1
Pollinator Garden Plant Suggestions - Perennials Heart of Uwchlan Project Tips for Planting a Pollinator Garden • Assess your location. Is it dry? Often wet? Is soil clay or loamy? How much sun or shade? Select plants appropriate to the conditions: “Right plant in the right place.” • Plant so you have blooms in every season. Don’t forget late summer/autumn bloomers; migrating butterflies need that late season pollen and nectar. • Plant for a variety of flower color and shape. That’s prettier for you, but it also appeals to a variety of pollinators. Some bees and butterflies prefer specific plants. • Plant in groups of at least three . easier for pollinators to find and browse. • Don’t forget the birds. Plant tubular flowers for hummingbirds, bushes with berries for birds (see related Plant List for Shrubs). • Finally, do minimal cleanup in the fall. Leave the leaves, dead stems and flower heads. Beneficial insects like miner bees lay eggs in hollow stems, finches will eat the echinacea seeds. Many butterflies and moths overwinter as pupae in dead leaves. Spring Blooming Golden-ragwort (Packera aurea) – mid to late Spring – Damp location, shade Grows freely and naturalizes into large colonies. Yellow flower heads, blooms for over 3 weeks in mide- to late spring. Dense ground cover. Prefers partial sun, medium shade. Prefers moist, swampy conditions. Cut back bloom stalks after flowering. Golden Alexander (Zizia aurea) – blooms May-June – prefers wet habitats but will tolerate dry Attractive bright yellow flower which occurs from May – June, looks like dill in shape. An excellent addition to a wildflower garden because it provides accessible nectar to many beneficial insects with short mouthparts during the spring and early summer when such flowers are relatively uncommon. -

Nomenclatural Studies Toward a World List of Diptera Genus-Group Names
Nomenclatural studies toward a world list of Diptera genus-group names. Part V Pierre-Justin-Marie Macquart Evenhuis, Neal L.; Pape, Thomas; Pont, Adrian C. DOI: 10.11646/zootaxa.4172.1.1 Publication date: 2016 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Evenhuis, N. L., Pape, T., & Pont, A. C. (2016). Nomenclatural studies toward a world list of Diptera genus- group names. Part V: Pierre-Justin-Marie Macquart. Magnolia Press. Zootaxa Vol. 4172 No. 1 https://doi.org/10.11646/zootaxa.4172.1.1 Download date: 02. Oct. 2021 Zootaxa 4172 (1): 001–211 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Monograph ZOOTAXA Copyright © 2016 Magnolia Press ISSN 1175-5334 (online edition) http://doi.org/10.11646/zootaxa.4172.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:22128906-32FA-4A80-85D6-10F114E81A7B ZOOTAXA 4172 Nomenclatural Studies Toward a World List of Diptera Genus-Group Names. Part V: Pierre-Justin-Marie Macquart NEAL L. EVENHUIS1, THOMAS PAPE2 & ADRIAN C. PONT3 1 J. Linsley Gressitt Center for Entomological Research, Bishop Museum, 1525 Bernice Street, Honolulu, Hawaii 96817-2704, USA. E-mail: [email protected] 2 Natural History Museum of Denmark, Universitetsparken 15, 2100 Copenhagen, Denmark. E-mail: [email protected] 3Oxford University Museum of Natural History, Parks Road, Oxford OX1 3PW, UK. E-mail: [email protected] Magnolia Press Auckland, New Zealand Accepted by D. Whitmore: 15 Aug. 2016; published: 30 Sept. 2016 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 NEAL L. -

Vascular Plants and a Brief History of the Kiowa and Rita Blanca National Grasslands
United States Department of Agriculture Vascular Plants and a Brief Forest Service Rocky Mountain History of the Kiowa and Rita Research Station General Technical Report Blanca National Grasslands RMRS-GTR-233 December 2009 Donald L. Hazlett, Michael H. Schiebout, and Paulette L. Ford Hazlett, Donald L.; Schiebout, Michael H.; and Ford, Paulette L. 2009. Vascular plants and a brief history of the Kiowa and Rita Blanca National Grasslands. Gen. Tech. Rep. RMRS- GTR-233. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 44 p. Abstract Administered by the USDA Forest Service, the Kiowa and Rita Blanca National Grasslands occupy 230,000 acres of public land extending from northeastern New Mexico into the panhandles of Oklahoma and Texas. A mosaic of topographic features including canyons, plateaus, rolling grasslands and outcrops supports a diverse flora. Eight hundred twenty six (826) species of vascular plant species representing 81 plant families are known to occur on or near these public lands. This report includes a history of the area; ethnobotanical information; an introductory overview of the area including its climate, geology, vegetation, habitats, fauna, and ecological history; and a plant survey and information about the rare, poisonous, and exotic species from the area. A vascular plant checklist of 816 vascular plant taxa in the appendix includes scientific and common names, habitat types, and general distribution data for each species. This list is based on extensive plant collections and available herbarium collections. Authors Donald L. Hazlett is an ethnobotanist, Director of New World Plants and People consulting, and a research associate at the Denver Botanic Gardens, Denver, CO. -

Species List For: Valley View Glades NA 418 Species
Species List for: Valley View Glades NA 418 Species Jefferson County Date Participants Location NA List NA Nomination and subsequent visits Jefferson County Glade Complex NA List from Gass, Wallace, Priddy, Chmielniak, T. Smith, Ladd & Glore, Bogler, MPF Hikes 9/24/80, 10/2/80, 7/10/85, 8/8/86, 6/2/87, 1986, and 5/92 WGNSS Lists Webster Groves Nature Study Society Fieldtrip Jefferson County Glade Complex Participants WGNSS Vascular Plant List maintained by Steve Turner Species Name (Synonym) Common Name Family COFC COFW Acalypha virginica Virginia copperleaf Euphorbiaceae 2 3 Acer rubrum var. undetermined red maple Sapindaceae 5 0 Acer saccharinum silver maple Sapindaceae 2 -3 Acer saccharum var. undetermined sugar maple Sapindaceae 5 3 Achillea millefolium yarrow Asteraceae/Anthemideae 1 3 Aesculus glabra var. undetermined Ohio buckeye Sapindaceae 5 -1 Agalinis skinneriana (Gerardia) midwestern gerardia Orobanchaceae 7 5 Agalinis tenuifolia (Gerardia, A. tenuifolia var. common gerardia Orobanchaceae 4 -3 macrophylla) Ageratina altissima var. altissima (Eupatorium rugosum) white snakeroot Asteraceae/Eupatorieae 2 3 Agrimonia pubescens downy agrimony Rosaceae 4 5 Agrimonia rostellata woodland agrimony Rosaceae 4 3 Allium canadense var. mobilense wild garlic Liliaceae 7 5 Allium canadense var. undetermined wild garlic Liliaceae 2 3 Allium cernuum wild onion Liliaceae 8 5 Allium stellatum wild onion Liliaceae 6 5 * Allium vineale field garlic Liliaceae 0 3 Ambrosia artemisiifolia common ragweed Asteraceae/Heliantheae 0 3 Ambrosia bidentata lanceleaf ragweed Asteraceae/Heliantheae 0 4 Ambrosia trifida giant ragweed Asteraceae/Heliantheae 0 -1 Amelanchier arborea var. arborea downy serviceberry Rosaceae 6 3 Amorpha canescens lead plant Fabaceae/Faboideae 8 5 Amphicarpaea bracteata hog peanut Fabaceae/Faboideae 4 0 Andropogon gerardii var. -

Barcoding the Asteraceae of Tennessee, Tribe Senecioneae
Schilling, E.E. and A. Floden. 2014. Barcoding the Asteraceae of Tennessee, tribe Senecioneae. Phytoneuron 2014-34: 1–5. Published 14 March 2014. ISSN 2153 733X BARCODING THE ASTERACEAE OF TENNESSEE, TRIBE SENECIONEAE EDWARD E. SCHILLING AND AARON FLODEN Herbarium TENN Department of Ecology & Evolutionary Biology University of Tennessee Knoxville, Tennessee 37996 [email protected]; [email protected] ABSTRACT Results from barcoding studies of tribe Senecioneae for the Tennessee flora using data from the nuclear ribosomal ITS marker region are presented and include first complete reports of this marker for 3 of the 15 species of these tribes that occur in the state. Sequence data from the ITS region separated all Tennessee species of Arnoglossum , Erechtites , Hasteola , and Rugelia (all of which are native) from one another and from other, non-Tennessee congeners. In contrast, many of the species of Packera , both from the state and from other parts of the southeastern USA, had basically identical ITS sequences. The contrast in the distinctiveness of Arnoglossum species compared to those of Packera suggests the two genera have had different histories of introduction and diversification in southeastern North America. Tribe Senecioneae is one of the largest in Asteraceae and with a worldwide distribution has had the opportunity to diversify in many different regions. The boundaries and circumscription of the tribe have, however, changed over the past few decades, and its generic level circumscription is still being settled (Nordenstam et al. 2009; Pelser et al. 2007, 2010). Notable is the problem of the circumscription of the huge Senecio (ca. 1000 species), but changes have also affected other genera from the southeastern USA, most notably the recognition of Arnoglossum and Hasteola as distinct from Cacalia (Anderson 1974). -
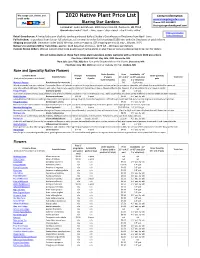
2020 Native Plant Price List
We accept cash, checks, and 2020 Native Plant Price List Contact information: credit cards: www.blazingstargardens.com Blazing Star Gardens Phone: 507-402-8337 [email protected] Located at: Souba Greenhouse, 4003 Crane Creek Rd, Owatonna, MN 55060 Greenhouse hours*: April - June, open 7 days a week - check hours online* *Hours and Location Retail Greenhouse: A limited selection of plants can be purchased daily at Souba's Greenhouse in Owatonna from April - June. Souba Greenhouse Full selection: To purchase from for our full selection, call or email to order for free pickup ($100 min. order) in Owatonna or paid delivery. Shipping across USA: (minimum order $100) We ship, costs are approx. $25 shipping per tray (1 tray = 18 pots, 3.5") Delivery to southern MN or Twin Cities: approx. $125 (less than 15 trays) - $275 (15 - 100 trays) per delivery Custom Grown Orders: We can custom grow large quantities of native plants in plug trays or pots at a discounted price. Call for details. We sell native plants at these Twin Cities plant sales (pre-orders welcome with a minimum $100 pre-order): Plant Sale: CANCELLED Sat. May 16th, 2020 Burnsville, MN Plant Sale: June TBD, 2020 9am-1pm at the Shepherd of the Hills Church, Shoreview, MN Plant Sale: June TBD, 2020 9am-1pm at Oakdale City Hall, Oakdale, MN Rare and Specialty Native Flowers Order Quantity Price Availability 3.5" Common Name Price per Availability Order Quantity Scientific Name 2" plants 3.5" or 4.5" or 4.5" pots (1 or Total Cost (click plants for pictures and details) 6-pack 6-packs pots (sold in 6-packs) pots 2 yr old plants) Monarch Factory Porch planter for monarchs - - $49 6 gallon pot Watch monarchs from your window! Our popular Monarch Factories are planted in large decorative pots and are perfect for porches or sidewalks, with plants that provide food for monarch caterpillars (Marsh Milkweed flowers), and nectar that attracts swarms of monarch butterflies in August (Meadow Blazing Star flowers). -

Bee Project Musing #3 July 27, 2018 Hello! This Week I'd Like to Tell You a Bit About Wasps, Stingers, Copycats and Sneaky Sp
Bee Project Musing #3 July 27, 2018 Hello! This week I’d like to tell you a bit about wasps, stingers, copycats and sneaky spiders and bees! Have you ever been stung by a bee or wasp? I had my most exciting wasp encounter when I was doing some work at my sister’s farm one summer. My siblings and I were transporting hay bales across the field and one bale happened to be the home of a Yellowjacket colony! I remember feeling a sharp sting on my ear followed by another dozen or so on my outstretched arms. This was followed by a chorus of alarmed cries of “Ow something bit me!” as my siblings and I fled the scene whooping and yelping as we ran. It was quite a painful and itchy experience, but we always laugh at ourselves when we think back to that day because it must have been a very funny scene to watch! Unlike most bees we encounter here in Toronto, some wasp species can be very territorial and aggressive like the Yellowjackets in the hay bale. The large yellow and black- striped flying insects pestering your picnic at the park are most likely Yellowjacket wasps. Most adult wasps survive on very sugary diets which is why you might see them on flowers (like a bee!) or on the rim of your sugary drink. Baby wasps (larvae) however, are often carnivorous. In some wasp species, Mother wasps catch and parasitize small caterpillars and grubs for their larvae to eat. In a separate bee project that I worked on last summer, I once opened up one of my bee hotel tubes to find it stuffed full of immobilized caterpillars! A window into a bee hotel tube shows the hotel full of caterpillars! I know wasps are sometimes thought to be the "bad guys" while bees are the "good guys", but I've come to love wasps all the same. -

Floristic Quality Assessment Report
FLORISTIC QUALITY ASSESSMENT IN INDIANA: THE CONCEPT, USE, AND DEVELOPMENT OF COEFFICIENTS OF CONSERVATISM Tulip poplar (Liriodendron tulipifera) the State tree of Indiana June 2004 Final Report for ARN A305-4-53 EPA Wetland Program Development Grant CD975586-01 Prepared by: Paul E. Rothrock, Ph.D. Taylor University Upland, IN 46989-1001 Introduction Since the early nineteenth century the Indiana landscape has undergone a massive transformation (Jackson 1997). In the pre-settlement period, Indiana was an almost unbroken blanket of forests, prairies, and wetlands. Much of the land was cleared, plowed, or drained for lumber, the raising of crops, and a range of urban and industrial activities. Indiana’s native biota is now restricted to relatively small and often isolated tracts across the State. This fragmentation and reduction of the State’s biological diversity has challenged Hoosiers to look carefully at how to monitor further changes within our remnant natural communities and how to effectively conserve and even restore many of these valuable places within our State. To meet this monitoring, conservation, and restoration challenge, one needs to develop a variety of appropriate analytical tools. Ideally these techniques should be simple to learn and apply, give consistent results between different observers, and be repeatable. Floristic Assessment, which includes metrics such as the Floristic Quality Index (FQI) and Mean C values, has gained wide acceptance among environmental scientists and decision-makers, land stewards, and restoration ecologists in Indiana’s neighboring states and regions: Illinois (Taft et al. 1997), Michigan (Herman et al. 1996), Missouri (Ladd 1996), and Wisconsin (Bernthal 2003) as well as northern Ohio (Andreas 1993) and southern Ontario (Oldham et al. -

Syrphidae of Southern Illinois: Diversity, Floral Associations, and Preliminary Assessment of Their Efficacy As Pollinators
Biodiversity Data Journal 8: e57331 doi: 10.3897/BDJ.8.e57331 Research Article Syrphidae of Southern Illinois: Diversity, floral associations, and preliminary assessment of their efficacy as pollinators Jacob L Chisausky‡, Nathan M Soley§,‡, Leila Kassim ‡, Casey J Bryan‡, Gil Felipe Gonçalves Miranda|, Karla L Gage ¶,‡, Sedonia D Sipes‡ ‡ Southern Illinois University Carbondale, School of Biological Sciences, Carbondale, IL, United States of America § Iowa State University, Department of Ecology, Evolution, and Organismal Biology, Ames, IA, United States of America | Canadian National Collection of Insects, Arachnids and Nematodes, Ottawa, Canada ¶ Southern Illinois University Carbondale, College of Agricultural Sciences, Carbondale, IL, United States of America Corresponding author: Jacob L Chisausky ([email protected]) Academic editor: Torsten Dikow Received: 06 Aug 2020 | Accepted: 23 Sep 2020 | Published: 29 Oct 2020 Citation: Chisausky JL, Soley NM, Kassim L, Bryan CJ, Miranda GFG, Gage KL, Sipes SD (2020) Syrphidae of Southern Illinois: Diversity, floral associations, and preliminary assessment of their efficacy as pollinators. Biodiversity Data Journal 8: e57331. https://doi.org/10.3897/BDJ.8.e57331 Abstract Syrphid flies (Diptera: Syrphidae) are a cosmopolitan group of flower-visiting insects, though their diversity and importance as pollinators is understudied and often unappreciated. Data on 1,477 Syrphid occurrences and floral associations from three years of pollinator collection (2017-2019) in the Southern Illinois region of Illinois, United States, are here compiled and analyzed. We collected 69 species in 36 genera off of the flowers of 157 plant species. While a richness of 69 species is greater than most other families of flower-visiting insects in our region, a species accumulation curve and regional species pool estimators suggest that at least 33 species are yet uncollected. -

Syrphidae (Diptera) of Northern Ontario and Akimiski Island, Nunavut: New Diversity Records, Trap Analysis, and DNA Barcoding
Syrphidae (Diptera) of northern Ontario and Akimiski Island, Nunavut: new diversity records, trap analysis, and DNA barcoding A Thesis Submitted to the Committee of Graduate Studies in Partial Fulfillment for the Degree of Master of Science in the Faculty of Arts and Science TRENT UNIVERSITY Peterborough, Ontario, Canada © Copyright by Kathryn A. Vezsenyi 2019 Environmental and Life Sciences M.Sc Graduate Program May 2019 ABSTRACT Syrphidae (Diptera) of northern Ontario and Akimiski Island, Nunavut: new diversity records, trap analysis, and DNA barcoding Kathryn A. Vezsenyi Syrphids, also known as hover flies (Diptera: Syrphidae) are a diverse and widespread family of flies. Here, we report on their distributions from a previously understudied region, the far north of Ontario, as well as Akimiski Island, Nunavut. I used samples collected through a variety of projects to update known range and provincial records for over a hundred species, bringing into clearer focus the distribution of syrphids throughout this region. I also analysed a previously un-tested trap type for collecting syrphids (Nzi trap), and report on results of DNA analysis for a handful of individuals, which yielded a potential new species. KEYWORDS Syrphidae, Diptera, insects, northern Ontario, Akimiski Island, diversity, insect traps, long term study, range extension, new species, DNA barcoding ii ACKNOWLEDGMENTS This thesis is written in dedication to my co-supervisor David Beresford, without whom none of this would have been possible. You have spent many long hours helping me with my data, my writing, and overall, my life. Your tireless, unwavering belief in me is one of the things that got me to where I am today, and has helped me grow as a person.