Water Quality Index Assessment of Ground Water in Koilwar Block of Bhojpur (Bihar)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Afu Srd Qoffir
qeio-ftocogofroelo/ lRMs- 10/2020, qi"...1.?.... fe-ax tcnffft-o gan frvn ffi (qrqrq s$rsq frqrrr) nso', sfi{r ts{ fu€r. frts o,d c-{ffi. ftErq crrfifto gun frvr ffi qe-+t ftr n, ftrm q-flDorfr. qfiIt qr.n. ftci--...a >ll.g.l.l..t:^ ftqq:- qrc-q ssrEFr c-{'Er{ c"nd (HRMS) clMn t-{r gkorot ovn rgqtqun-sqa iD't Scanning d tidq { t q{frl:- fr{H qr qiw-''titz. fr{ifr-1s.io.2o1s aefl q{i6-gol. fr{is- 29.05.2020 IT6ITq, sqEffi frqq-o' crciFro q'it d snr enc si-{q-d t fu rrq ofid oI \1otq-d srcrn{ d-fi oa qFit qa or eR-o G crrflff $ffiq-qf, qr+q \4qErt !-qtrr cqrfr (HRMs) ffi( o-{i d mq d fu S*Zo* qms q'r Digitisation aeTI e-tar gw ot ffiu fu sqcIcr qtrqr"qt (legacy oata) fr Data capture Format (DCFIi cfre o'-{i or ord srt i t 2. -cniFro' c=jl n +sI gRilor. ridl= 3IRs st 6ffi{it ErqI sqnrq orri rrc Efrqw q, ol Scanning 6{i d €dtl I arerrl i] oTd-6q +1 qaw frn qri ot s-de fu-ql .rqr t t q-c-{ o. tfl Sfu"ol r{ €dcun qr d scanning t-E ftrd d orufa-q-qR aTtroq frt q.ff *ia.{ tr (3r-flff-r) 4- Scanning 61 o,rd qqF-( 1'Ss lvs CBSL eRI ffiqr .rfi. f,a, 3Tq{ qfoq. .rfi Fmfur fuTFr d ory S qmi srd o.rt it ftcio-oa.o'r.zoz'r d qrtqt"-gef ro:oo ad t 3rq{rfl 6:00 cd ao ria.c o'd-6'q F-5{ff-r) o r5wn fu-qI qrvqrt 5. -

Final English Version of E-Bulletin (6
Embassy of India 6 - 12 Dec 2020 Jakarta INDIA'S LATEST NEWS NEWS MISSION'S ACTIVITIES HIGHLIGHT Agra, City of Taj Mahal Metro project inaugurated; Nitin Gadkari Inaugurates Three- Lane Koilwar Bridge on Sone River Worth Rs 266 Crore; Bhagalpur address for winged guests; #India@75 Efficient new method of harvesting water from air developed by IIT Guwahati; Lakshadweep declared first UT to become 100% organic. : indianembassyjakarta.gov.in : IndianEmbJkt : IndiaInIndonesia : Embassy of India Jakarta 1 Agra, City of Taj Mahal Metro project inaugurated Prime Minister Narendra Modi inaugurated the construction work of Agra Metro project in Agra, Uttar Pradesh through video conference. The metro project comprised of two corridors with a total length of 29.4 km and is estimated to cost Rs 8,3796.2 Million. The project will cater to more than 6 million tourists and 2.6 million residents of Agra every year. It will also provide an environment-friendly mass rapid transit system to Agra. The project will connect major tourist attractions like Taj Mahal, Agra Fort, Sikandra with railway stations and bus stands and it will be completed in five years. The metro in Agra will run at a speed of 80 Kmph and the cost of one metro rail of three coaches is pegged at Rs 80 Million. Source: Times Now Nitin Gadkari Inaugurates Three-Lane Koilwar Bridge on Sone River Worth Rs 266 Crore Union Minister for Road Transport and Highways, Nitin Gadkari, inaugurated the three-lane Koilwar bridge on the Sone river in Bihar through video conference. The bridge costing Rs 266 crore would make commuting easier in South Bihar. -

East Central Railway
10.1.1 पूव मय रेलवे EAST CENTRAL RAILWAY 20192019----2020 के िलए पƗरसंपिēयĪ कƙ खरीद , िनमाϕण और बदलाव Assets-Acquisition, Construction and Replacement for 2019-20 (Figures in thousand of Rupees)(आंकड़े हजार Đ . मĞ) पूंजी पूंजी िनिध मूआिन िविन संिन रारेसंको जोड़ िववरण Particulars Capital CF DRF. DF SF RRSK TOTAL 11 (a) New Lines (Construction) 248,00,00 .. .. .. 200,00,00 .. 448,00,00 14 G Gauge Conversion 185,00,00 .. .. .. .. .. 185,00,00 15 ह Doubling 10,00,00 .. .. .. .. .. 10,00,00 16 - G Traffic Facilities-Yard 16,46,33 .. .. 3,05,00 .. 8,82,18 28,33,51 G ^ G Remodelling & Others 17 Computerisation 2,01,00 .. 5,00,00 57,50 .. .. 7,58,50 21 Rolling Stock 16,80,00 .. .. 1,75,00 .. 13,20,00 31,75,00 22 * 4 - Leased Assets - Payment 511,32,64 202,97,36 .. .. .. .. 714,30,00 of Capital Component 29 E G - Road Safety Works-Level .. .. .. .. .. 59,28,48 59,28,48 Crossings. 30 E G -/ Road Safety Works-Road .. .. .. .. .. 144,62,00 144,62,00 Over/Under Bridges. 31 Track Renewals .. .. .. .. .. 634,84,29 634,84,29 32 G Bridge Works 4,35,00 .. .. .. .. 51,95,90 56,30,90 33 G Signalling and 4,00 .. .. .. .. 132,40,02 132,44,02 Telecommunication 36 ^ G - G Other Electrical Works 29,07,31 .. 2,03,84 1,97,81 .. 78,22 33,87,18 K excl TRD 37 G G Traction Distribution 1,00,00 .. .. .. .. 84,69,86 85,69,86 Works 41 U Machinery & Plant 4,16,35 . -

Bhojpur District, Bihar State
भूजल सूचना पुस्तिका भोजपुर स्जला, बिहार Ground Water Information Booklet Bhojpur District, Bihar State 84° 15' 84° 30' 84° 45' BIHAR STATE ADMINISTRATIVE MAP N BHOJPUR DISTRICT, BIHAR. Ganga R. 25° 25° 45' 45' GANGA RIVER Barhara Gaura Bhagar N. Dehra N. Shahpur Gangi N. Koilwar Chher N. Bihiya ARA 25° Udawant 25° nagar 30' Gangi N. 30' Jagdishpur Garhani KumhariSandes N. Charpokhari Agiaon Banas N. Piro 25° Sahar 25° 15' SON RIVER 15' LEGEND Tarari District Boundary Block Boundary River District HQ. 0 5 10 15 20 Km Block HQ. Road Scale Railway 84° 15' 84° 30' 84° 45' के न्द्रीय भमू िजल िो셍 ड Central Ground water Board Ministry of Water Resources जल संसाधन िंत्रालय (Govt. of India) (भारि सरकार) Mid-Eastern Region िध्य-पर्वू ी क्षेत्र Patna पटना मसिंिर 2013 September 2013 1 PREPARED BY - Shri S. Sahu Sc. B UNDER SUPERVISION OF - Shri A.K.Agrawal, Scientist’D’ CARTOGRAPHY - Shri Lokendra Kumar, D/Man Gd-II 2 UPDATED BY - Shri S.N.Dwivedi, Sc- C & Dr. Fakhre Alam, STA (Hg) CONTENTS DISTRICT AT A GLANCE. 5-6 1. INTRODUCTION 7 - 10 1.1 Location, Area and Administrative Details 1.2 Basin/Sub-Basin and Drainage 1.3 Landuse, Agriculture and Irrigation Practices 1.4 Studies/Activities carried by CGWB 2. CLIMATE AND RAINFALL 10 3. GEOMORPHOLOGY AND SOIL 10-11 3.1 Geomorphology 3.2 Soil 4. GROUND WATER SCENARIO 11 - 17 4.1 Water Bearing Formations 4.2 Occurrence & Movement of Ground Water 4.3 Depth to Water Level 4.4 Ground Water Quality 4.4.1 Arsenic in Ground Water 4.5 Ground Water Resources 5. -

Bhojpur 2019-20
Ministry of Micro, Small & Medium Enterprises Government of India DISTRICT PROFILE BHOJPUR 2019-20 Carried out by MSME-Development Institute (Ministry of MSME, Govt. of India,) Patliputra Industrial Estate, Patna-13 Phone:- 0612-2262719, 2262208, 2263211 Fax: 06121 -2262186 e-mail: [email protected] Web- www.msmedipatna.gov.in Veer Kunwar Singh Memorial, Ara, Bhojpur Sun Temple, Tarari, Bhojpur 2 FOREWORD At the instance of the Development Commissioner, Micro, Small & Medium Enterprises, Government of India, New Delhi, District Industrial Profile containing basic information about the district of Bhojpur has been updated by MSME-DI, Patna under the Annual Plan 2019-20. It covers the information pertaining to the availability of resources, infrastructural support, existing status of industries, institutional support for MSMEs, etc. I am sure this District Industrial Profile would be highly beneficial for all the Stakeholders of MSMEs. It is full of academic essence and is expected to provide all kinds of relevant information about the District at a glance. This compilation aims to provide the user a comprehensive insight into the industrial scenario of the district. I would like to appreciate the relentless effort taken by Shri Ravi Kant, Assistant Director (EI) in preparing this informative District Industrial Profile right from the stage of data collection, compilation upto the final presentation. Any suggestion from the stakeholders for value addition in the report is welcome. Place: Patna Date: 31.03.2020 3 Brief Industrial Profile of Bhojpur District 1. General Characteristics of the District– Bhojpur district was carved out of erstwhile Shahbad district in 1992. The Kunwar Singh, the leader of the Mutineers during Sepoy Mutiny in 1857, was from district Bhojpur. -
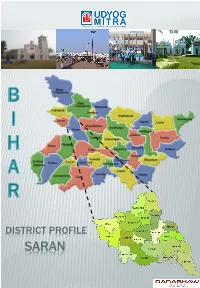
Saran Introduction
DISTRICT PROFILE SARAN INTRODUCTION Saran district is one of the thirty-eight districts of Bihar. Saran district is a part of Saran division. Saran district is also known as Chhapra district because the headquarters of this district is Chhapra. Saran district is bounded by the districts of Siwan, Gopalganj, West Champaran, Muzaffarpur, Patna, Vaishali and Bhojpur of Bihar and Ballia district of Uttar Pradesh. Important rivers flowing through Saran district are Ganga, Gandak, and Ghaghra which encircle the district from south, north east and west side respectively. HISTORICAL BACKGROUND Saran was earlier known as ‘SHARAN’ which means refuge in English, after the name given to a Stupa (pillar) built by Emperor Ashoka. Another view is that the name Saran has been derived from SARANGA- ARANYA or the deer forest since the district was famous for its forests and deer in pre-historic times. In ancient days, the present Saran division, formed a part of Kosala kingdom. According to 'Ain-E-Akbari’, Saran was one of the six Sarkars/ revenue divisions, constituting the province of Bihar. By 1666, the Dutch established their trade in saltpetre at Chhapra. Saran was one of the oldest and biggest districts of Bihar. In 1829, Saran along with Champaran, was included in the Patna Division. Saran was separated from Champaran in 1866 when Champaran district was constituted. In 1981, the three subdivisions of the old Saran district namely Saran, Siwan and Gopalganj became independent districts which formed a part of Saran division. There are a few villages in Saran which are known for their historical and social significance. -
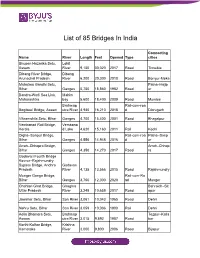
List of 85 Bridges in India
List of 85 Bridges In India Connecting Name River Length Feet Opened Type cities Bhupen Hazarika Setu, Lohit Assam River 9,150 30,020 2017 Road Tinsukia Dibang River Bridge, Dibang Arunachal Pradesh River 6,200 20,300 2018 Road Bomjur-Meka Mahatma Gandhi Setu, Patna–Hajip Bihar Ganges 5,750 18,860 1982 Road ur Bandra-Worli Sea Link, Mahim Maharashtra bay 5,600 18,400 2009 Road Mumbai Brahmap Rail-cum-roa Bogibeel Bridge, Assam utra River 4,940 16,210 2018 d Dibrugarh Vikramshila Setu, Bihar Ganges 4,700 15,400 2001 Road Bhagalpur Vembanad Rail Bridge, Vembana Kerala d Lake 4,620 15,160 2011 Rail Kochi Digha–Sonpur Bridge, Rail-cum-roa Patna–Sonp Bihar Ganges 4,556 14,948 2016 d ur Arrah–Chhapra Bridge, Arrah–Chhap Bihar Ganges 4,350 14,270 2017 Road ra Godavari Fourth Bridge Kovvur–Rajahmundry Bypass Bridge, Andhra Godavari Pradesh River 4,135 13,566 2015 Road Rajahmundry Munger Ganga Bridge, Rail-cum-Ro Bihar Ganges 3,750 12,300 2020 ad Munger Chahlari Ghat Bridge, Ghaghra Bahraich–Sit Uttar Pradesh River 3,249 10,659 2017 Road apur Jawahar Setu, Bihar Son River 3,061 10,043 1965 Road Dehri Nehru Setu, Bihar Son River 3,059 10,036 1900 Rail Dehri Kolia Bhomora Setu, Brahmap Tezpur–Kalia Assam utra River 3,015 9,892 1987 Road bor Korthi-Kolhar Bridge, Krishna Karnataka River 3,000 9,800 2006 Road Bijapur Netaji Subhas Chandra Kathajodi Bose Setu, Odisha River 2,880 9,450 2017 Road Cuttack Godavari Bridge, Andhra Godavari Rail-cum-roa Pradesh River 2,790 1974 d Rajahmundry Old Godavari Bridge Now decommissioned, Godavari Andhra Pradesh -

BIHAR SPECIAL Current Affairs (December 2020)
www.gradeup.co BIHAR SPECIAL Current Affairs (December 2020) Bollywood actor Sanjay Mishra becomes brand ambassador of Patna Municipal Corporation • The people of Bihar to make the state capital clean and beautiful, Bollywood’s famous comedy actor Sanjay Mishra has been declared the brand ambassador of Patna Municipal Corporation. Actor Sanjay Mishra who is going to convey the message, “Beauty of Patna is the Duty of Patna” among the people the state capital. Kabartal Wetland of Begusarai included in the Ramsar list • Ramsar Convention declared Kabartal Wetland also known as Kanwar Jheel in Begusarai, Bihar as a site of international importance. It covers 2,620 hectares of the Indo-Gangetic plains in the northern Bihar State. • Kabartal has become the first in Bihar and the 39th Ramsar site in India. This lake was given the status of bird sanctuary by the Bihar government in 1987. EC Appoints First Transgender Woman as Presiding Officer for Bihar Polls • Monika Das, is the first-ever transgender person to be appointed as a presiding poll officer in independent India’s history. Her selection is a big victory for the transgender community, which can often be underrepresented in public life. • For the past five years, she has been working at a bank, currently employed with Canara Bank. Prior to this, Monica has also become the country's first transgender banker. Fisheries and animal husbandry schemes inaugurated in Bihar • Prime Minister Shri Narendra Modi launched PM Matsya Sampada Yojana, e- Gopala App & several initiatives linked to studies and research in fisheries production, dairy, animal husbandry and agriculture in Bihar through video conferencing. -

TACR: India: Preparing the Bihar State Highways II Project
Technical Assistance Consultant’s Report Project Number: 41629 October 2010 India: Preparing the Bihar State Highways II Project Prepared by Sheladia Associates, Inc. Maryland, USA For Road Construction Department Government of Bihar This consultant’s report does not necessarily reflect the views of ADB or the Government concerned, and ADB and the Government cannot be held liable for its contents. (For project preparatory technical assistance: All the views expressed herein may not be incorporated into the proposed project’s design. TA No. 7198-INDIA: Preparing the Bihar State Highways II Project Final Report TTTAAABBBLLLEEE OOOFFF CCCOOONNNTTTEEENNNTTTSSS 1 INTRODUCTION .............................................................................................................................. 8 1.1 INTRODUCTION .............................................................................................................................. 8 1.2 PROJECT APPRECIATION............................................................................................................. 8 1.2.1 Project Location and Details 9 1.2.2 Road Network of Bihar 11 1.3 PERFORMANCE OF THE STUDY ................................................................................................ 14 1.3.1 Staff Mobilization 14 1.3.2 Work Shop 14 1.4 STRUCTURE OF THE FINAL REPORT ....................................................................................... 14 2 SOCIO ECONOMIC PROFILE OF PROJECT AREA .................................................................. -
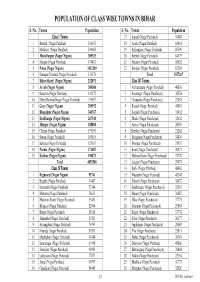
Population of Class Wise Towns in Bihar
POPULATION OF CLASS WISE TOWNS IN BIHAR S. No. Towns Population S. No. Towns Population Class I Towns 17 Supaul (Nagar Parishad) 54085 1 Bettiah (Nagar Parishad) 116670 18 Araria (Nagar Parishad) 60861 2 Motihari (Nagar Parishad) 100683 19 Kishanganj (Nagar Parishad) 85590 3 Muzaffarpur (Nagar Nigam) 305525 20 Beehat (Nagar Parishad) 64579 4 Hajipur (Nagar Parishad) 119412 21 Barauni (Nagar Parishad) 58628 5 Patna (Nagar Nigam) 1432209 22 Benipur (Nagar Parishad) 62203 6 Danapur Nizamat (Nagar Parishad) 131176 Total 1557267 7 Bihar Sharif (Nagar Nigam) 232071 Class III Towns 8 Arrah (Nagar Nigam) 203380 1 Narkatiaganj (Nagar Parishad) 40830 9 Sasaram (Nagar Parishad) 131172 2 Ramnagar (Nagar Panchayat) 38554 10 Dehri DalmiyaNagar (Nagar Parishad) 119057 3 Chanpatia (Nagar Panchayat) 22038 11 Gaya (Nagar Nigam) 389192 4 Raxaul (Nagar Parishad) 41610 12 Bhagalpur (Nagar Nigam) 340767 5 Sugauli (Nagar Panchayat) 31432 13 Darbhanga (Nagar Nigam) 267348 6 Dhaka (Nagar Panchayat) 32632 14 Munger (Nagar Nigam) 188050 7 Areraj (Nagar Panchayat) 20356 15 Chapra (Nagar Parishad) 179190 8 Sheohar (Nagar Panchayat) 21262 16 Siwan (Nagar Parishad) 109919 9 Bairgania (Nagar Panchayat) 34836 17 Saharsa (Nagar Parishad) 125167 10 Motipur (Nagar Panchayat) 21957 18 Purnia (Nagar Nigam) 171687 11 Kanti (Nagar Panchayat) 20871 19 Katihar (Nagar Nigam) 190873 12 Mahnar Bazar (Nagar Panchayat) 37370 Total 4853548 13 Lalganj (Nagar Panchayat) 29873 Class II Towns 14 Barh (Nagar Parishad) 48442 1 Begusarai (Nagar Nigam) 93741 15 Masaurhi (Nagar Parishad) 45248 -

The Report of the Patna Iniversity Committee
CRITICISMS ON THE REPORT OF THE PATNA INIVERSITY COMMITTEE. T o D - t i < f |- 37S>-^STr-1sV'' i ' N l ^ C ¥ * l U - C . CRITICISMS ON THE REPORT OF THE PATNA UNIVEE SITY COMMITTEE; TABLE OF CONTENTS. P a g e Babu Badri Nath Upadhya, Korha, Pumea ... 1 Mr. P. Walfordj Principal, Bihar School of Engineering , 3 Rai Upendra Nath Ghosh, Bahadur, Deputy Collector in charge 6 of Orissa Canals, Revenue Division, iCuttack. Bihar Planters'Association ... ... ... 11 The National Society, Balasore ... ... 12 Mr. E. Schroder, Headmaster, Zila School, Bhagalpur , 13 Church Missionary Society, Calcutta ... ... , 16 Bihar Provincial Moslem League, Bankipore ... , 17 Ranchi Bihari Public ... ... ... 19 Bengali Settlers’ Association, Bhagalpur ... 20 Hon’ble Babu Bishun Prasad ... 22 Dr. Lakshmipati ... ... ... , 25 Mr. R. N . Gilchrist, Professor, Presidency College, Calcutta . 29 Mahamahopadhya Pandit Ganga Nath Jha 32 Hon’ble Maulvi Saiyid Muhammad Tahir ... 33 Edward Memorial Ayurvedic Pathshala, Aurangabad, Gaya , 36 Muhammadan Association, Bhagalpur ... ... 38 Moslem League, Bhagalpur ... ... • •• < 39 Patna Bar Association, Bankipore cat I 40 Fifth Bihar Provincial Conference ... ... 47, 63 Bengali Settlers' Association, Bihar and Orissa, Bankipore , 53 Commissioner, Bhagalpur Division ... ... 55 District Magistrate, Monghyr _ ' ' ^ ... 55 Commissioner, Patna Division ... 59 District Magistrate, Shahabad ... ... 59 Khan Sahib Saiyid Ahmad Ali Khan ••• I 60 Snbdivisional OfSoer, Buxar ... ... 61 Subdivisional Officer, Sasaram ... 61 District Magistrate, Gaya ... ... ... 61 Hon'ble Maharaja Sir Ravaneswar Praead Singh, Bahadnr, . 62 k .c.i j :.. of Gidhaur. Muhammadans of Chota Nagpur ,.l ... • 66 Commissioner, Orissa Division ... ... • 67 District Magistrate, Balasore ... ••• • 68, Pasgb District Magistrate, P’atna ... 71 Malthil Mahasabha, Darblianga 73, 86 Mr. S. Q. Huda ... ... 74 Calcutta Weekly Notes .. -

SH-88: Barunapul-Rasiyari Subproject, Bihar State Highways II Project
Initial Environmental Examination November 2011 IND: Bihar State Highways II Project – Additional Financing (SH-88: Barunapul-Rasiyari Subproject) Prepared by Bihar State Road Development Corporation Limited for the Asian Development Bank. CURRENCY EQUIVALENTS (as of 30 November 2011) Currency unit – Indian rupee (Rs) INR1.00 = $0.0192104505 $1.00 = INR52.055000 ABBREVIATIONS ADB – Asian Development Bank BSHPII- – Bihar State Highways Project II- Additional Financing AF BSRDC – Bihar State Road Development Corporation Ltd. BSPCB – Bihar State Pollution Control Board CGM – Chief General Manager CPCB – Central Pollution Control Board CRRI – Central Road Research Institute DoE – Department of Environment DFO – District Forest Officer DGM – Deputy General Manager DPR – Detailed Project Report EA – Executing Agency EFRC – Environment Friendly Road Construction EIA – Environmental Impact Assessment EMP – Environmental Management Plan EMoP – Environmental Management and Monitoring Plan EO – Environmental Officer GDP – Gross Domestic Product GoI – Government of India GoB – Government of Bihar GRC – Grievance Redressal Mechanism IEE – Initial Environmental Examination IMD – Indian Meteorological Department INR – Indian Rupees IPP – Indigenous Peoples Plan IRC – Indian Road Congress MDRs – Major District Roads MoEF – Ministry of Environment and Forests MoSRTH – Ministry of Shipping, Road Transport and Highways MSL – Mean Sea Level NGO – Non Government Organization NH – National Highway NOx – Oxides of Nitrogen NSDP – Net State Domestic Product