Motor Neuron Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinically Undetected Motor Neuron Disease in Pathologically Proven Frontotemporal Lobar Degeneration with Motor Neuron Disease
ORIGINAL CONTRIBUTION Clinically Undetected Motor Neuron Disease in Pathologically Proven Frontotemporal Lobar Degeneration With Motor Neuron Disease Keith A. Josephs, MST, MD; Joseph E. Parisi, MD; David S. Knopman, MD; Bradley F. Boeve, MD; Ronald C. Petersen, MD, PhD; Dennis W. Dickson, MD Background: Frontotemporal lobar degeneration with evidence of motor neuron disease. Semiquantitative motor neuron disease (FTLD-MND) is a pathological analysis of motor and extramotor pathological findings entity characterized by motor neuron degeneration and revealed a spectrum of pathological changes underlying frontotemporal lobar degeneration. The ability to detect FTLD-MND. Hippocampal sclerosis, predominantly of the clinical signs of dementia and motor neuron disease the subiculum, was a significantly more frequent occur- in pathologically confirmed FTLD-MND has not been rence in the cases without clinical evidence of motor assessed. neuron disease (PϽ.01). In addition, neuronal loss, gliosis, and corticospinal tract degeneration were less Objectives: To determine if all cases of pathologically severe in the other 3 cases without clinical evidence of confirmed FTLD-MND have clinical evidence of fronto- motor neuron disease. temporal dementia and motor neuron disease, and to de- termine the possible reasons for misdiagnosis. Conclusions: Clinical diagnostic sensitivity for the el- ements of FTLD-MND is modest and may be affected by Method: Review of historical records and semiquantita- the fact that FTLD-MND represents a spectrum of patho- tive analysis of the motor and extramotor pathological find- logical findings, rather than a single homogeneous en- ings of all cases of pathologically confirmed FTLD-MND. tity. Detection of signs of clinical motor neuron disease is also difficult when motor neuron degeneration is mild Results: From a total of 17 cases of pathologically con- and in patients with hippocampal sclerosis. -

Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia
brain sciences Review Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia Timothy Fullam and Jeffrey Statland * Department of Neurology, University of Kansas Medical Center, Kansas, KS 66160, USA; [email protected] * Correspondence: [email protected] Abstract: Following the exclusion of potentially reversible causes, the differential for those patients presenting with a predominant upper motor neuron syndrome includes primary lateral sclerosis (PLS), hereditary spastic paraplegia (HSP), or upper motor neuron dominant ALS (UMNdALS). Differentiation of these disorders in the early phases of disease remains challenging. While no single clinical or diagnostic tests is specific, there are several developing biomarkers and neuroimaging technologies which may help distinguish PLS from HSP and UMNdALS. Recent consensus diagnostic criteria and use of evolving technologies will allow more precise delineation of PLS from other upper motor neuron disorders and aid in the targeting of potentially disease-modifying therapeutics. Keywords: primary lateral sclerosis; amyotrophic lateral sclerosis; hereditary spastic paraplegia Citation: Fullam, T.; Statland, J. Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper 1. Introduction Motor Neuron Dominant Jean-Martin Charcot (1825–1893) and Wilhelm Erb (1840–1921) are credited with first Amyotrophic Lateral Sclerosis, and describing a distinct clinical syndrome of upper motor neuron (UMN) tract degeneration in Hereditary Spastic Paraplegia. Brain isolation with symptoms including spasticity, hyperreflexia, and mild weakness [1,2]. Many Sci. 2021, 11, 611. https:// of the earliest described cases included cases of hereditary spastic paraplegia, amyotrophic doi.org/10.3390/brainsci11050611 lateral sclerosis, and underrecognized structural, infectious, or inflammatory etiologies for upper motor neuron dysfunction which have since become routinely diagnosed with the Academic Editors: P. -

ALS2CR2 (STRADB) 406-418) Goat Polyclonal Antibody – AP08962PU-N
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for AP08962PU-N ALS2CR2 (STRADB) 406-418) Goat Polyclonal Antibody Product data: Product Type: Primary Antibodies Applications: ELISA, IHC, WB Recommended Dilution: ELISA: 1/32000. Immunohistochemistry on Paraffin Sections: 3.75 µg/ml. Western Blot: 1 - 3 µg/ml. Reactivity: Canine, Human Host: Goat Clonality: Polyclonal Immunogen: Synthetic peptide from C-terminus of human ALS2CR2 Specificity: This antibody reacts to STE20-Related Kinase Adaptor Beta (STRADB/ALS2CR2) at aa 406-418. It is expected to recognise both human isoforms: ILPIP-alpha (NP_061041.2) and ILPIP-beta (AAF71042.1). Formulation: Tris saline buffer, pH 7.3, 0.5% BSA, 0.02% sodium azide State: Aff - Purified State: Liquid purified Ig Concentration: lot specific Purification: Immunoaffinity Chromatography Conjugation: Unconjugated Storage: Store the antibody undiluted at 2-8°C for one month or (in aliquots) at -20°C for longer. Avoid repeated freezing and thawing. Stability: Shelf life: one year from despatch. Database Link: Entrez Gene 55437 Human Q9C0K7 This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 3 ALS2CR2 (STRADB) 406-418) Goat Polyclonal Antibody – AP08962PU-N Background: Amyotrophic lateral sclerosis 2 (juvenile) chromosome region, candidate 2, is connected to transferase/kinase activity and ATP binding, it has recently been shown to interact with XIAP, a member of the IAP (Inhibitor of Apoptosis) protein family. -

Neural Control of Movement: Motor Neuron Subtypes, Proprioception and Recurrent Inhibition
List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Mem- ic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, Eichele G, Whelan PJ, Kullander K (2010) Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as mark- ers for fast motor neurons and partition cells. J Comp Neurol 518:2284-2304. II Wootz H, Enjin A, Wallen-Mackenzie Å, Lindholm D, Kul- lander K (2010) Reduced VGLUT2 expression increases motor neuron viability in Sod1G93A mice. Neurobiol Dis 37:58-66 III Enjin A, Leao KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K. 5-ht1d marks gamma motor neurons and regulates development of sensorimotor connections Manuscript IV Enjin A, Leao KE, Eriksson A, Larhammar M, Gezelius H, Lamotte d’Incamps B, Nagaraja C, Kullander K. Development of spinal motor circuits in the absence of VIAAT-mediated Renshaw cell signaling Manuscript Reprints were made with permission from the respective publishers. Cover illustration Carousel by Sasha Svensson Contents Introduction.....................................................................................................9 Background...................................................................................................11 Neural control of movement.....................................................................11 The motor neuron.....................................................................................12 Organization -

ALS and Other Motor Neuron Diseases Can Represent Diagnostic Challenges
Review Article Address correspondence to Dr Ezgi Tiryaki, Hennepin ALS and Other Motor County Medical Center, Department of Neurology, 701 Park Avenue P5-200, Neuron Diseases Minneapolis, MN 55415, [email protected]. Ezgi Tiryaki, MD; Holli A. Horak, MD, FAAN Relationship Disclosure: Dr Tiryaki’s institution receives support from The ALS Association. Dr Horak’s ABSTRACT institution receives a grant from the Centers for Disease Purpose of Review: This review describes the most common motor neuron disease, Control and Prevention. ALS. It discusses the diagnosis and evaluation of ALS and the current understanding of its Unlabeled Use of pathophysiology, including new genetic underpinnings of the disease. This article also Products/Investigational covers other motor neuron diseases, reviews how to distinguish them from ALS, and Use Disclosure: Drs Tiryaki and Horak discuss discusses their pathophysiology. the unlabeled use of various Recent Findings: In this article, the spectrum of cognitive involvement in ALS, new concepts drugs for the symptomatic about protein synthesis pathology in the etiology of ALS, and new genetic associations will be management of ALS. * 2014, American Academy covered. This concept has changed over the past 3 to 4 years with the discovery of new of Neurology. genes and genetic processes that may trigger the disease. As of 2014, two-thirds of familial ALS and 10% of sporadic ALS can be explained by genetics. TAR DNA binding protein 43 kDa (TDP-43), for instance, has been shown to cause frontotemporal dementia as well as some cases of familial ALS, and is associated with frontotemporal dysfunction in ALS. Summary: The anterior horn cells control all voluntary movement: motor activity, res- piratory, speech, and swallowing functions are dependent upon signals from the anterior horn cells. -

Motor Neuron Disease and the Elderly
Neurology 61 Motor neuron disease and the elderly Motor neuron disease is a devastating condition characterised by degeneration of motor nerves. Many of the presenting symptoms, such as fatigue, muscle weakness and difficulty in swallowing have a broad differential diagnoses in the elderly population. Dr Sheba Azam and Professor PN Leigh explain how ensuring quality of life for patients requires preventing unnecessary delay in diagnosis and early referral to an appropriate multidisciplinary team. myotrophic lateral sclerosis (ALS) also cognitive changes. Thus, misdiagnosis as well as known as motor neuron disease (MND) under-investigation has been suggested as possible (the terms are used interchangeably), was causes of an apparent decrease in the incidence of A 4 fi rst described in 1869 by the French neurologist MND in later life . Jean-Martin Charcot1. It is a progressive, fatal neurological disease characterised by degeneration of motor nerve cells in the motor cortex, Prognostic factors corticospinal tract and the spinal cord anterior horn Although the average survival in MND is around cells. The degeneration of motor nerve cells results 36 months, some patients live for 10 years or more. in progressive muscle wasting leading to signifi cant Certain phenotypic variants appear to determine disability and ultimately death. Death usually survival rates. Using information held in a tertiary results from respiratory failure due to weakness of referral MND database, a group of researchers the respiratory muscles. analysed data on onset of disease, site of onset and duration of survival5. The authors concluded that typical MND with bulbar onset, onset later in life Incidence and prevalence or in the defi nite category of El Escorial (where The worldwide incidence of MND is approximately the World Federation of Neurologist meet to a professor of clinical two per 100,000 and the prevalence is four to seven decide diagnostic criteria) at presentation, per 100,000. -

A System for Studying Mechanisms of Neuromuscular Junction Development and Maintenance Valérie Vilmont1,‡, Bruno Cadot1, Gilles Ouanounou2 and Edgar R
© 2016. Published by The Company of Biologists Ltd | Development (2016) 143, 2464-2477 doi:10.1242/dev.130278 TECHNIQUES AND RESOURCES RESEARCH ARTICLE A system for studying mechanisms of neuromuscular junction development and maintenance Valérie Vilmont1,‡, Bruno Cadot1, Gilles Ouanounou2 and Edgar R. Gomes1,3,*,‡ ABSTRACT different animal models and cell lines (Chen et al., 2014; Corti et al., The neuromuscular junction (NMJ), a cellular synapse between a 2012; Lenzi et al., 2015) with the hope of recapitulating some motor neuron and a skeletal muscle fiber, enables the translation of features of neuromuscular diseases and understanding the triggers chemical cues into physical activity. The development of this special of one of their common hallmarks: the disruption of the structure has been subject to numerous investigations, but its neuromuscular junction (NMJ). The NMJ is one of the most complexity renders in vivo studies particularly difficult to perform. studied synapses. It is formed of three key elements: the lower motor In vitro modeling of the neuromuscular junction represents a powerful neuron (the pre-synaptic compartment), the skeletal muscle (the tool to delineate fully the fine tuning of events that lead to subcellular post-synaptic compartment) and the Schwann cell (Sanes and specialization at the pre-synaptic and post-synaptic sites. Here, we Lichtman, 1999). The NMJ is formed in a step-wise manner describe a novel heterologous co-culture in vitro method using rat following a series of cues involving these three cellular components spinal cord explants with dorsal root ganglia and murine primary and its role is basically to ensure the skeletal muscle functionality. -

Cranial Nerves 1, 5, 7-12
Cranial Nerve I Olfactory Nerve Nerve fiber modality: Special sensory afferent Cranial Nerves 1, 5, 7-12 Function: Olfaction Remarkable features: – Peripheral processes act as sensory receptors (the other special sensory nerves have separate Warren L Felton III, MD receptors) Professor and Associate Chair of Clinical – Primary afferent neurons undergo continuous Activities, Department of Neurology replacement throughout life Associate Professor of Ophthalmology – Primary afferent neurons synapse with secondary neurons in the olfactory bulb without synapsing Chair, Division of Neuro-Ophthalmology first in the thalamus (as do all other sensory VCU School of Medicine neurons) – Pathways to cortical areas are entirely ipsilateral 1 2 Crania Nerve I Cranial Nerve I Clinical Testing Pathology Anosmia, hyposmia: loss of or impaired Frequently overlooked in neurologic olfaction examination – 1% of population, 50% of population >60 years Aromatic stimulus placed under each – Note: patients with bilateral anosmia often report nostril with the other nostril occluded, eg impaired taste (ageusia, hypogeusia), though coffee, cloves, or soap taste is normal when tested Note that noxious stimuli such as Dysosmia: disordered olfaction ammonia are not used due to concomitant – Parosmia: distorted olfaction stimulation of CN V – Olfactory hallucination: presence of perceived odor in the absence of odor Quantitative clinical tests are available: • Aura preceding complex partial seizures of eg, University of Pennsylvania Smell temporal lobe origin -

Lancl1 Promotes Motor Neuron Survival and Extends the Lifespan of Amyotrophic Lateral Sclerosis Mice
Cell Death & Differentiation (2020) 27:1369–1382 https://doi.org/10.1038/s41418-019-0422-6 ARTICLE LanCL1 promotes motor neuron survival and extends the lifespan of amyotrophic lateral sclerosis mice 1 1 1 1 1 1 1 Honglin Tan ● Mina Chen ● Dejiang Pang ● Xiaoqiang Xia ● Chongyangzi Du ● Wanchun Yang ● Yiyuan Cui ● 1,2 1 1,3 4 4 3 1,5 Chao Huang ● Wanxiang Jiang ● Dandan Bi ● Chunyu Li ● Huifang Shang ● Paul F. Worley ● Bo Xiao Received: 19 November 2018 / Revised: 3 September 2019 / Accepted: 6 September 2019 / Published online: 30 September 2019 © The Author(s) 2019. This article is published with open access Abstract Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by progressive loss of motor neurons. Improving neuronal survival in ALS remains a significant challenge. Previously, we identified Lanthionine synthetase C-like protein 1 (LanCL1) as a neuronal antioxidant defense gene, the genetic deletion of which causes apoptotic neurodegeneration in the brain. Here, we report in vivo data using the transgenic SOD1G93A mouse model of ALS indicating that CNS-specific expression of LanCL1 transgene extends lifespan, delays disease onset, decelerates symptomatic progression, and improves motor performance of SOD1G93A mice. Conversely, CNS-specific deletion of fl 1234567890();,: 1234567890();,: LanCL1 leads to neurodegenerative phenotypes, including motor neuron loss, neuroin ammation, and oxidative damage. Analysis reveals that LanCL1 is a positive regulator of AKT activity, and LanCL1 overexpression restores the impaired AKT activity in ALS model mice. These findings indicate that LanCL1 regulates neuronal survival through an alternative mechanism, and suggest a new therapeutic target in ALS. -

Novel Pathomechanisms in Inflammatory Neuropathies David Schafflick1, Bernd C
Schafflick et al. Journal of Neuroinflammation (2017) 14:232 DOI 10.1186/s12974-017-1001-8 REVIEW Open Access Novel pathomechanisms in inflammatory neuropathies David Schafflick1, Bernd C. Kieseier2, Heinz Wiendl1 and Gerd Meyer zu Horste1* Abstract Inflammatory neuropathies are rare autoimmune-mediated disorders affecting the peripheral nervous system. Considerable progress has recently been made in understanding pathomechanisms of these disorders which will be essential for developing novel diagnostic and therapeutic strategies in the future. Here, we summarize our current understanding of antigenic targets and the relevance of new immunological concepts for inflammatory neuropathies. In addition, we provide an overview of available animal models of acute and chronic variants and how new diagnostic tools such as magnetic resonance imaging and novel therapeutic candidates will benefit patients with inflammatory neuropathies in the future. This review thus illustrates the gap between pre-clinical and clinical findings and aims to outline future directions of development. Keywords: Experimental autoimmune neuritis, Guillain-Barré syndrome, Chronic inflammatory demyelinating polyneuropathy, Animal model, Th17 cells Background between 0.8 and 1.9 per 100,000 worldwide. Men are Inflammatory neuropathies are a heterogeneous group of approximately 1.5-fold more frequently affected and the autoimmune disorders affecting the peripheral nervous incidence increases with age [6]. Although the majority of system (PNS) that can exhibit an acute or chronic disease patients recover, the prognosis can remain poor with severe course. They are rare, but cause significant and often per- disability or death in 9–17% of all GBS patients [7]. In many manent disability in many affected patients [1, 2]. -
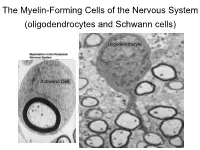
The Myelin-Forming Cells of the Nervous System (Oligodendrocytes and Schwann Cells)
The Myelin-Forming Cells of the Nervous System (oligodendrocytes and Schwann cells) Oligodendrocyte Schwann Cell Oligodendrocyte function Saltatory (jumping) nerve conduction Oligodendroglia PMD PMD Saltatory (jumping) nerve conduction Investigating the Myelinogenic Potential of Individual Oligodendrocytes In Vivo Sparse Labeling of Oligodendrocytes CNPase-GFP Variegated expression under the MBP-enhancer Cerebral Cortex Corpus Callosum Cerebral Cortex Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Ant Commissure Corpus Callosum Cerebral Cortex Caudate Putamen Piriform Cortex Corpus Callosum Ant Commissure Characterization of Oligodendrocyte Morphology Cerebral Cortex Corpus Callosum Caudate Putamen Cerebellum Brain Stem Spinal Cord Oligodendrocytes in disease: Cerebral Palsy ! CP major cause of chronic neurological morbidity and mortality in children ! CP incidence now about 3/1000 live births compared to 1/1000 in 1980 when we started intervening for ELBW ! Of all ELBW {gestation 6 mo, Wt. 0.5kg} , 10-15% develop CP ! Prematurely born children prone to white matter injury {WMI}, principle reason for the increase in incidence of CP ! ! 12 Cerebral Palsy Spectrum of white matter injury ! ! Macro Cystic Micro Cystic Gliotic Khwaja and Volpe 2009 13 Rationale for Repair/Remyelination in Multiple Sclerosis Oligodendrocyte specification oligodendrocytes specified from the pMN after MNs - a ventral source of oligodendrocytes -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body.