Toxic Cyanobacteria in Water; a Guide to Their Public Health Consequences, Monitoring and Management; Second Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protocols for Monitoring Harmful Algal Blooms for Sustainable Aquaculture and Coastal Fisheries in Chile (Supplement Data)
Protocols for monitoring Harmful Algal Blooms for sustainable aquaculture and coastal fisheries in Chile (Supplement data) Provided by Kyoko Yarimizu, et al. Table S1. Phytoplankton Naming Dictionary: This dictionary was constructed from the species observed in Chilean coast water in the past combined with the IOC list. Each name was verified with the list provided by IFOP and online dictionaries, AlgaeBase (https://www.algaebase.org/) and WoRMS (http://www.marinespecies.org/). The list is subjected to be updated. Phylum Class Order Family Genus Species Ochrophyta Bacillariophyceae Achnanthales Achnanthaceae Achnanthes Achnanthes longipes Bacillariophyta Coscinodiscophyceae Coscinodiscales Heliopeltaceae Actinoptychus Actinoptychus spp. Dinoflagellata Dinophyceae Gymnodiniales Gymnodiniaceae Akashiwo Akashiwo sanguinea Dinoflagellata Dinophyceae Gymnodiniales Gymnodiniaceae Amphidinium Amphidinium spp. Ochrophyta Bacillariophyceae Naviculales Amphipleuraceae Amphiprora Amphiprora spp. Bacillariophyta Bacillariophyceae Thalassiophysales Catenulaceae Amphora Amphora spp. Cyanobacteria Cyanophyceae Nostocales Aphanizomenonaceae Anabaenopsis Anabaenopsis milleri Cyanobacteria Cyanophyceae Oscillatoriales Coleofasciculaceae Anagnostidinema Anagnostidinema amphibium Anagnostidinema Cyanobacteria Cyanophyceae Oscillatoriales Coleofasciculaceae Anagnostidinema lemmermannii Cyanobacteria Cyanophyceae Oscillatoriales Microcoleaceae Annamia Annamia toxica Cyanobacteria Cyanophyceae Nostocales Aphanizomenonaceae Aphanizomenon Aphanizomenon flos-aquae -
Supporting Information
1 Supporting Information 2 Contents: 3 Table S1 : TOC-MAR and OC gross sedimentation data from four lakes page S-1 4 Table S2 : Fred and TOC MAR values of six selected lakes page S-1 5 Figure S1 : Porewater profiles from Lake Zug page S-2 6 Figure S2 : Seasonal development of O2 concentration page S-3 7 8 9 Table S1: Average fluxes of TOC MAR, TOC gross sedimentation and the corresponding OC burial efficiency based on sediment trap data. TOC MAR at deepest benthic gross OC Burial Monitoring duration, Sampling Lake point sedimentation ref effiency % month-year interval gC m-2 yr-1 gC m-2 yr-1 Lake 43.79 45.62 104.19 4-2013 to 11-2014 2 weeks Baldegg Lake Aegeri 77.45 22.77 29.40 3-2014 to 12-2014 2 weeks Lake Hallwil 41.59 22.51 54.12 1-2014 to 12-2014 monthly Lake Rene Gächter 45.96 28.00 60.92 1-1984 to 12-1992 varying Sempach unpublished 10 11 12 Table S2: Characteristics of three eutrophic, one mesotrophic, and two oligotrophic lakes. Fred data for Rotsee, Türlersee, Lake Sempach, Lake 13 Murten and Pfäffikersee are from Müller et al. (2012) and Fred was calculated for Lake Erie (Adams et al., 1982), Lake Superior (Richardson 14 and Nealson, 1989; Remsen et al., 1989; Klump et al., 1989; Heinen and McManus, 2004; Li et al., 2012), and Lake Baikal (Och et al., 2012). 15 TOC MAR was calculated for all lakes based on literature data: Lake Murten (Müller and Schmid, 2009), Lake Baikal (Och et al., 2012), Lake 16 Sempach (Müller et al., 2012), Rotsee (RO) (Naeher et al., 2012), Pfäffikersee (unpublished data), Türlersee (Matzinger et al., 2008), Lake Erie 17 (Smith and Matisoff, 2008; Matisoff et al., 1977) and Lake Superior (Klump et al., 1989; Li et al., 2012). -

Planktothrix Agardhii É a Mais Comum
Accessing Planktothrix species diversity and associated toxins using quantitative real-time PCR in natural waters Catarina Isabel Prata Pereira Leitão Churro Doutoramento em Biologia Departamento Biologia 2015 Orientador Vitor Manuel de Oliveira e Vasconcelos, Professor Catedrático Faculdade de Ciências iv FCUP Accessing Planktothrix species diversity and associated toxins using quantitative real-time PCR in natural waters The research presented in this thesis was supported by the Portuguese Foundation for Science and Technology (FCT, I.P.) national funds through the project PPCDT/AMB/67075/2006 and through the individual Ph.D. research grant SFRH/BD65706/2009 to Catarina Churro co-funded by the European Social Fund (Fundo Social Europeu, FSE), through Programa Operacional Potencial Humano – Quadro de Referência Estratégico Nacional (POPH – QREN) and Foundation for Science and Technology (FCT). The research was performed in the host institutions: National Institute of Health Dr. Ricardo Jorge (INSA, I.P.), Lisboa; Interdisciplinary Centre of Marine and Environmental Research (CIIMAR), Porto and Centre for Microbial Resources (CREM - FCT/UNL), Caparica that provided the laboratories, materials, regents, equipment’s and logistics to perform the experiments. v FCUP Accessing Planktothrix species diversity and associated toxins using quantitative real-time PCR in natural waters vi FCUP Accessing Planktothrix species diversity and associated toxins using quantitative real-time PCR in natural waters ACKNOWLEDGMENTS I would like to express my gratitude to my supervisor Professor Vitor Vasconcelos for accepting to embark in this research and supervising this project and without whom this work would not be possible. I am also greatly thankful to my co-supervisor Elisabete Valério for the encouragement in pursuing a graduate program and for accompanying me all the way through it. -

Change of Phytoplankton Composition and Biodiversity in Lake Sempach Before and During Restoration
Hydrobiologia 469: 33–48, 2002. S.A. Ostroumov, S.C. McCutcheon & C.E.W. Steinberg (eds), Ecological Processes and Ecosystems. 33 © 2002 Kluwer Academic Publishers. Printed in the Netherlands. Change of phytoplankton composition and biodiversity in Lake Sempach before and during restoration Hansrudolf Bürgi1 & Pius Stadelmann2 1Department of Limnology, ETH/EAWAG, CH-8600 Dübendorf, Switzerland E-mail: [email protected] 2Agency of Environment Protection of Canton Lucerne, CH-6002 Lucerne, Switzerland E-mail: [email protected] Key words: lake restoration, biodiversity, evenness, phytoplankton, long-term development Abstract Lake Sempach, located in the central part of Switzerland, has a surface area of 14 km2, a maximum depth of 87 m and a water residence time of 15 years. Restoration measures to correct historic eutrophication, including artificial mixing and oxygenation of the hypolimnion, were implemented in 1984. By means of the combination of external and internal load reductions, total phosphorus concentrations decreased in the period 1984–2000 from 160 to 42 mg P m−3. Starting from 1997, hypolimnion oxygenation with pure oxygen was replaced by aeration with fine air bubbles. The reaction of the plankton has been investigated as part of a long-term monitoring program. Taxa numbers, evenness and biodiversity of phytoplankton increased significantly during the last 15 years, concomitant with a marked decline of phosphorus concentration in the lake. Seasonal development of phytoplankton seems to be strongly influenced by the artificial mixing during winter and spring and by changes of the trophic state. Dominance of nitrogen fixing cyanobacteria (Aphanizomenon sp.), causing a severe fish kill in 1984, has been correlated with lower N/P-ratio in the epilimnion. -

Microcystin Incidence in the Drinking Water of Mozambique: Challenges for Public Health Protection
toxins Review Microcystin Incidence in the Drinking Water of Mozambique: Challenges for Public Health Protection Isidro José Tamele 1,2,3 and Vitor Vasconcelos 1,4,* 1 CIIMAR/CIMAR—Interdisciplinary Center of Marine and Environmental Research, University of Porto, Terminal de Cruzeiros do Porto, Avenida General Norton de Matos, 4450-238 Matosinhos, Portugal; [email protected] 2 Institute of Biomedical Science Abel Salazar, University of Porto, R. Jorge de Viterbo Ferreira 228, 4050-313 Porto, Portugal 3 Department of Chemistry, Faculty of Sciences, Eduardo Mondlane University, Av. Julius Nyerere, n 3453, Campus Principal, Maputo 257, Mozambique 4 Faculty of Science, University of Porto, Rua do Campo Alegre, 4069-007 Porto, Portugal * Correspondence: [email protected]; Tel.: +351-223-401-817; Fax: +351-223-390-608 Received: 6 May 2020; Accepted: 31 May 2020; Published: 2 June 2020 Abstract: Microcystins (MCs) are cyanotoxins produced mainly by freshwater cyanobacteria, which constitute a threat to public health due to their negative effects on humans, such as gastroenteritis and related diseases, including death. In Mozambique, where only 50% of the people have access to safe drinking water, this hepatotoxin is not monitored, and consequently, the population may be exposed to MCs. The few studies done in Maputo and Gaza provinces indicated the occurrence of MC-LR, -YR, and -RR at a concentration ranging from 6.83 to 7.78 µg L 1, which are very high, around 7 times · − above than the maximum limit (1 µg L 1) recommended by WHO. The potential MCs-producing in · − the studied sites are mainly Microcystis species. -

Population Dynamics of Whitefish ( Coregonus Suidteri Fatio) in Artificially Oxygenated Lake Hallwil, with Special Emphasis on L
Diss. ETH No. 13706 Population dynamics of whitefish ( Coregonus suidteri Fatio) in artificially oxygenated Lake Hallwil, with special emphasis on larval mortality and sustainable management Dissertation submitted to the SWISS FEDERAL INSTITUTE OF TECHNOLOGY, ZURICH for the degree of Doctor of Natural Sciences presented by Carole Andrea Enz Dipl. Natw. ETH Zurich bon1 August 3, 1972 Citizen of Sch(1nholzerswilen (TG), Switzerland accepted on the recommendation of Prof. Dr. J. V. Ward, examiner Prof. Dr. H. Lehtonen, co-examiner Dr. R. Muller, co-examiner Kastanienbaum, 2000 Meinen Eltern und Max Copyright ~;i 2000 by Carole A. Enz, EA WAG Kastanienbaurn All rights reserved. No part of this book rnay be reproduced, stored in a retrieval systen1 or transmitted, in any fonn or by any ineans, electronic, rnechanical, pho- tocopying, recording or otherwise, without the prior written permission of the copyright holder. First Edition 2000 PUBLICATIONS CHAPTER 3 OF THE THESIS HAS BEEN ACCEPTED FOR PUBLICATION: ENZ, C. A., SCHAFFER E. & MOLLER R. Growth and survival of Lake Hallwil whitefish (Co reg onus suidteri) larvae reared on dry and live food. - Archiv fUr Hyclrobiologie. CHAPTERS 4, 5, 6 Ai~D 7 OF THESIS HA VE BEEN SUBMITTED FOR PUBLICATION: ENZ, C. A., MBWENEMO BIA, M. & MULLER. R. Fish species diversity of Lake Hallwil (Switzerland) in the course of eutrophication, with special reference to whitefish ( Coregonus suidteri). Submitted to Conservation Biology. ENZ, C. A .. SCHAFFER, E. & MULLER, R. Importance of prey movement, food particle and tank circulation for rearing Lake Ha11wil whitefish (Coregonus suidteri) larvae. Submitted to North Alnerican Journal of Aquaculture. -
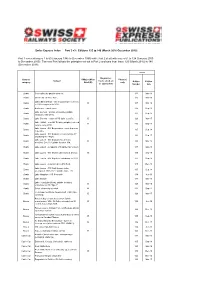
Swiss Express Index Part 3 V3 : Editions 125 to 140 (March 2016-December 2019)
Swiss Express Index Part 3 v3 : Editions 125 to 140 (March 2016-December 2019) Part 1 covered issues 1 to 60 (January 1985 to December 1999) while Part 2 dealt with issues 61 to 124 (January 2000 to December 2015). This new Part follows the principles set out in Part 2 and runs from Issue 125 (March 2016) to 140 (December 2019). Issue Diagram(s) - General If Major (M) or Photo(s) Subject track, stock etc category Brief (B) only Edition Edition as appropriate Number date Boats Commuting by paddle steamer. 133 Mar-18 Boats DS St Urs on River Aare 125 Mar-16 Lakes Brienz/Thun - rise in passenger numbers Boats B 137 Mar-19 in 2018 compared to 2017. Boats Bodensee Train Ferries 139 Sep-19 Lake Geneva - parade of (mostly) paddle Boats 127 Sep-16 steamers, May 2016. Boats Lake Geneva - return of PS Italie to traffic. B 129 Mar-17 Lake Hallwil - new MV Delphin (dolphin) entered Boats B 135 Sep-18 service June 2018. Lake Luzern - MV Bürgenstock - new ship now Boats 135 Sep-18 in service. Lake Luzern - MV Diamant in service May 17 Boats 131 Sep-17 (replacing the "Rigi"). Lake Luzern - MV Diamant holed in an Boats B 133 Mar-18 accident, Dec.18. Update in Issue 134. Boats Lake Luzern - scrapping of freighter MV Luzern 137 Mar-19 Boats Lake Luzern - MV Mythen damaged at Gersau. B 135 Sep-18 Boats Lake Luzern - MV Rigi to be withdrawn in 2017. 127 Sep-16 Boats Lake Luzern - A quick trip on MV Rütli. -

Predictive Modeling of Microcystin Concentrations in Drinking Water Treatment Systems Of
Predictive Modeling of Microcystin Concentrations in Drinking Water Treatment Systems of Ohio and their Potential Health Effects Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By: Traven A. Wood, B.S. Graduate Program in Public Health The Ohio State University 2019 Thesis Committee: Mark H. Weir (Adviser) Jiyoung Lee Allison MacKay Copyright by Traven Aldin Wood 2019 Abstract Cyanobacteria present significant public health and engineering challenges due to their expansive growth and potential synthesis of microcystins in surface waters that are used as a drinking water source. Eutrophication of surface waters coupled with favorable climatic conditions can create ideal growth environments for these organisms to develop what is known as a cyanobacterial harmful algal bloom (cHAB). Development of methods to predict the presence and impact of microcystins in drinking water treatment systems is a complex process due to system uncertainties. This research developed two predictive models, first to estimate microcystin concentrations at a water treatment intake, second, to estimate the risks of finished water detections after treatment and resultant health effects to consumers. The first model uses qPCR data to adjust phycocyanin measurements to improve predictive linear regression relationships. Cyanobacterial 16S rRNA and mcy genes provide a quantitative means of measuring and detecting potentially toxic genera/speciess of a cHAB. Phycocyanin is a preferred predictive tool because it can be measured in real-time, but the drawback is that it cannot distinguish between toxic genera/speciess of a bloom. Therefore, it was hypothesized that genus specific ratios using qPCR data could be used to adjust phycocyanin measurements, making them more specific to the proportion of the bloom that is producing toxin. -

(Oscillatoriales: Phormidiaceae) in Earthen Fish Ponds, Northwestern
Sains Malaysiana 41(3)(2012): 277–284 Dynamics of Cyanobacteria Planktothrix species (Oscillatoriales: Phormidiaceae) in Earthen Fish Ponds, Northwestern Bangladesh (Kedinamikan Planktothrix spesies (Oscillatoriales: Phormidiaceae) dalam Kolam Ikan Bertanah Liat, Barat Laut Bangladesh) MD. YEAMIN HOSSAIN*, MD. ABU SAYED JEWEL, BERNERD FULANDA, FERDOUS AHAMED, SHARMEEN RAHMAN, SALEHA JASMINE & JUN OHTOMI ABSTRACT The seasonal abundance, dynamics and composition of the filamentous Cyanobacteria Planktothrix spp. was studied over a 1-year period in two storm-water-fed earthen fishponds in Rajshahi city, northwestern Bangladesh. Sampling was conducted monthly using plankton net (25 µm mesh size) and the samples preserved in 5% formalin. Water quality parameters including water temperature, transparency, pH, dissolved oxygen (DO), biological oxygen demand (BOD), + free carbon dioxide (CO2), nitrite-nitrogen (NO2-N), nitrate-nitrogen (NO3-N), ammonium (NH4 ), oxidation reduction index (rH2) were recorded during each sampling. Two species; Planktothrix agardhii and Planktothrix rubescens were identified during the study with P. agardhii recording higher abundance (p<0.05) all year round. The Planktothrix cell density was highest during March: 3.06×106 cells/L and 1.23×106 cells/L in Pond-1 and 2, respectively. The abundance of P. agardhii was relatively higher in spring. The cell densities increased with increasing temperature, pH, and nutrient concentration. Lower cell densities were recorded during periods of high BOD. The results of this study provide a useful guide for aquaculturists and other environmental scientists for the management of the cyanotoxin producing algal blooms of Planktothrix spp. in fertilized fish ponds and other aquatic habitats. Keywords: Bangladesh; earthen fish pond;Planktothrix ; seasonal dynamics; storm run-off ABSTRAK Kelimpahan, kedinamikan dan komposisi bermusim Cyanobacteria Planktothrix spp. -

Organic Carbon Mass Accumulation Rate Regulates the Flux of Reduced Substances from the Sediments of Deep Lakes
Research Collection Journal Article Organic carbon mass accumulation rate regulates the flux of reduced substances from the sediments of deep lakes Author(s): Steinsberger, Thomas; Schmid, Martin; Wüest, Alfred; Schwefel, Robert; Wehrli, Bernhard; Müller, Beat Publication Date: 2017-07-10 Permanent Link: https://doi.org/10.3929/ethz-b-000191117 Originally published in: Biogeosciences 14(13), http://doi.org/10.5194/bg-14-3275-2017 Rights / License: Creative Commons Attribution 3.0 Unported This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Biogeosciences, 14, 3275–3285, 2017 https://doi.org/10.5194/bg-14-3275-2017 © Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License. Organic carbon mass accumulation rate regulates the flux of reduced substances from the sediments of deep lakes Thomas Steinsberger1,2, Martin Schmid1, Alfred Wüest1,3, Robert Schwefel3, Bernhard Wehrli1,2, and Beat Müller1 1Eawag, Swiss Federal Institute of Aquatic Science and Technology, 6047 Kastanienbaum, Switzerland 2Institute of Biogeochemistry and Pollutant Dynamics, ETH Zurich, 8092 Zurich, Switzerland 3Physics of Aquatic Systems Laboratory, Margaretha Kamprad Chair, École Polytechnique Fédérale de Lausanne, Institute of Environmental Engineering, 1015 Lausanne, Switzerland Correspondence to: Beat Müller ([email protected]) Received: 31 January 2017 – Discussion started: 17 February 2017 Revised: 23 May 2017 – Accepted: 2 June 2017 – Published: 10 July 2017 Abstract. The flux of reduced substances, such as methane hypolimnetic O2 consumption (Livingstone and Imboden, and ammonium, from the sediment to the bottom water (Fred/ 1996; Hutchinson, 1938; Cornett and Rigler, 1980), yet the is one of the major factors contributing to the consumption key processes are still debated. -

Sales Manual. Swiss Travel System
Sales Manual. Swiss Travel System. Version 1, 2020 Swiss Travel Guide Button-PRINT.pdf 1 20.08.19 14:27 Go digital – get the app! English Bernina at Lago Bianco Express STS-GB-L-20-en.pdfSTS-GB-L-20-en.pdfSTS-GB-L-20-en.pdf 1 1 18.09.19 18.09.19 11:301 11:30 18.09.19 11:30 StrasbourgStrasbourg | Paris | StrasbourgParis | Paris KarlsruheKarlsruhe | Frankfurt |Karlsruhe Frankfurt | Dortmund | | FrankfurtDortmund | Hamburg | Dortmund | Hamburg | Berlin | Hamburg| Berlin | Berlin Stuttgart StuttgartStuttgart Ulm | MünchenUlmUlm | München | München München MünchenMünchen Stockach StockachStockach Engen Engen Engen Blumberg-ZollhausBlumberg-ZollhausBlumberg-Zollhaus DEUTSCHLANDDEUTSCHLANDDEUTSCHLAND SeebruggSeebruggSeebrugg Bargen BargenOpfertshofenBargen Opfertshofen Ravensburg RavensburgRavensburg Train,Train,Train, busbus and andbus boat boatand boat Opfertshofen Überlingen ÜberlingenÜberlingen BeggingenBeggingenBeggingen Singen SingenSingen Thayngen ThayngenThayngen HemmentalHemmentalHemmental atat aa glanceglanceat a glance Rhein/Rhein/ Rhein/ SchleitheimSchleitheimSchleitheim MulhouseMulhouse Mulhouse Radolfzell RadolfzellRadolfzell Le RhinLe Rhin Le Rhin Mainau MainauMainau Version:Version: 12.20 12.201919 Version: 12.2019 Meersburg MeersburgMeersburg DueDue to to lack lack of of space spaceDue not not toall alllack lines lines of are spaceare indicated. indicated. not all Subjectlines Subject are to indicated. tochange. change. Subject to change. SchaffhausenSchaffhausenSchaffhausenRamsen RamsenRamsen Wangen (Allgäu)WangenWangen (Allgäu) -

Bacterial Communities in the Glenmore Reservoir with an Emphasis on the Cyanobacteria
Bacterial Communities in the Glenmore Reservoir with an emphasis on the Cyanobacteria by Ghadeer Aljoudi A thesis presented to the University of Waterloo in fulfillment of the thesis requirement for the degree of Master of Science In Biology Waterloo, Ontario, Canada, 2021 © Ghadeer Aljoudi 2021 Author’s Declaration I hereby declare that I am the sole author of this thesis. This is a true copy of the thesis, including any required final revisions, as accepted by my examiners. I understand that my thesis may be made electronically available to the public. ii Abstract Lakes and reservoirs play an essential role as a source for freshwater that can be potable after proper treatment, or for irrigation to meet the water needs of the population, industry, and agriculture. However, freshwaters bodies face significant challenges (including pollutant loads and climate change) that may lead to water quality degradation; among these are taste and odour events and harmful bacterial blooms. In Calgary, Alberta, Canada, runoff from agriculture and residential development in urbanized watersheds also contributes to water quality deterioration in the Elbow River, which flows into the Glenmore Reservoir and serves as one of the primary sources of drinking water for the city. While harmful algal blooms (i.e., Cyanobacteria) have not occurred in the Glenmore Reservoir historically, increased pressures (e.g., stormwater discharges, potential erosion and runoff after wildfire) on source water quality in the city’s wildfire-prone source watersheds have the potential to increase nutrient (especially bioavailable phosphorus) loading to the reservoir; this can promote algal blooms (City of Calgary, 2018).