WO 2016/102490 Al 30 June 2016 (30.06.2016) W P O P C T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report of a Mermithid Nematode Infecting Amyna Axis, Chrysodeixisspp. and Spodoptera Spp. from India
Journal of Biological Control, 33(3): 217-221,2019, DOI: 10.18311/jbc/2019/22801 Research Article Report of a mermithid nematode infecting Amyna axis, Chrysodeixis spp. and Spodoptera spp. from India S. RAMESH BABU1, VICTOR PHANI2, P. K. MEENA1, KHUSHBU CHAUHAN2, M. R. KHAN2 and V. S. SOMVANSHI2* 1Agriculture Research Station, Maharana Pratap University of Agriculture and Technology, Banswara - 327001, Rajasthan, India 2Division of Nematology, ICAR-Indian Agricultural Research Institute, PUSA Campus, New Delhi - 110012, India *Corresponding author E-mail: [email protected]; [email protected] ABSTRACT: Amyna axis (Lepidoptera: Erebidae), Chrysodeixis spp. (C. acuta and C. eriosoma) and Spodoptera spp. (S. exigua and S. litura) (Lepidoptera: Noctuidae) are important polyphagous insect-pests of leguminous crops. A mermithid (Nematoda: Mermithidae) nematode was found parasitizing the larval stages of these insects in the western Indian state of Rajasthan. Morphological and molecular analysis suggests that the nematode might be a species of the genus Hexamermis. The average length of post-parasitic nematode juveniles was 15 cm, and the average greatest body width was 1.5 mm. The vulva was median (V% = 48) without vulval flap. Approximately 100-125 µm long caudal appendages were present on tail. The cross-section revealed the presence of six hypodermal chords at the mid body region, and stichosome was present. The 18S rDNA sequence showed 99% similarity to mermithid nematode. A phylogenetic analysis of 18S rDNA sequences of mermithids resulted in identification of a new clade “Lineage 3” which included representatives of Mermis sp., Isomermis sp., Limnomermis sp., and Pheromermis sp. This is the first report of natural mermithid parasitism of A. -

Time September 27
Time September 27 - Thursday 08:00 Registration 09:00 09:00 Inaugural Session 10:00 10:00 Refreshment Break 10:30 10:30 Opening lecture (Coronet Hall) 11:00 Why so many manuscripts are rejected for publication? An editorial perspective - Quirico Migheli Time Coronet Hall Senate Hall Time Orchid Hall Session 1: Biodiversity, Biosecurity and Session 2: Biotechnological Approaches in Session 8: Third International Workshop of the Conservation Strategies Biocontrol IOBC Global Working Group on Biological Control and Management of Parthenium Weed 11:00 Biodiversity bugs pests: An ecological Small insecticidal molecules against crop 11:00 Welcome & Housekeeping - Lorraine Strathie 11:30 perspective-Krishna Kumar N K pests- Raj Kamal Bhatnagar 11:10 11:30 Biosystematics, biodiversity and biocontrol Role of transgenic Bt-crops in promoting 11:10 Opening of Parthenium Weed Workshop - 12:00 relationships-Ramamurthy VV biological control and integrated pest 11:20 Rangaswamy Muniappan management- Manjunath T M 12:00 Yeast volatile organic compounds inhibit 11:20 Keynote address – Biological control of 12:15 Pest management services through ochratoxin - A production by Aspergillus 11:50 parthenium weed (Parthenium hysterophorus L.): conservation of biological control agents: carbonarius (Bainier)- Quirico Migheli Australian experience - Kunjithapatham Dhileepan, review, case studies and field experiences- Boyang Shi, Jason Callander and Segun Osunkoya 12:15 Abraham Verghese Silencing of a Meloidogyne incognita mucin- Theme: Spread and impact of Parthenium 12:30 like gene reduces Pasteuria penetrans hysterophorus (Chair: Lorraine Strathie) endospore adhesion on the juvenile cuticle surface - Uma Rao 11:50 Nationwide survey of parthenium weed infestation 12:05 in Bangladesh: risk and threat for food security. -

Coral Sea CMR 2016, a Bush Blitz Survey Report
Coral Sea Commonwealth Marine Reserve 13–24 June 2016 Bush Blitz Species Discovery Program CSCMR 13–24 June 2016 What is Bush Blitz? Bush Blitz is a multi-million dollar partnership between the Australian Government, BHP Billiton Sustainable Communities and Earthwatch Australia to document plants and animals in selected conservation areas across Australia. This innovative partnership harnesses the expertise of many of Australia’s top scientists from museums, herbaria, universities, and other institutions and organisations across the country. Abbreviations ABRS Australian Biological Resources Study ANIC Australian National Insect Collection ATH Australian Tropical Herbarium AVH Australia’s Virtual Herbarium CSCMR Coral Sea Commonwealth Marine Reserve EPBC Act Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth) IUCN International Union for Conservation of Nature QM Queensland Museum UNSW University of New South Wales WAM Western Australian Museum Page 2 of 32 CSCMR 13–24 June 2016 Summary The Coral Sea Bush Blitz took place between 13 and 24 June 2016. Four islands of the Coral Sea Commonwealth Marine Reserve (CSCMR) were surveyed: East Diamond Islet, South West Coringa Islet, North East Herald Cay and South West Herald Cay, including the surrounding coral reefs. This Bush Blitz was undertaken in partnership with the Commonwealth Marine Reserves Branch of Parks Australia as part of the Great Coral Sea Clean-up and Bio-Discovery Voyage. The partnership aimed to develop a better understanding of marine debris, flora and fauna, and quarantine issues in the CSCMR. In the survey, 175 taxa were collected. The reserve’s flora and fauna has an inherently low biodiversity largely comprising widespread species that have broad Indo-Pacific or pan-tropical distribution patterns. -

Butterflies and Moths of North America
Heliothis ononis Flax Bollworm Moth Coptotriche aenea Blackberry Leafminer Argyresthia canadensis Habrosyne scripta Lettered Habrosyne Habrosyne gloriosa Glorious Habrosyne Pseudothyatira cymatophoroides Tufted Thyatirid Thyatira mexicana Euthyatira lorata Euthyatira semicircularis Ceranemota improvisa Ceranemota fasciata Ceranemota crumbi Ceranemota semifasciata Ceranemota tearlei Ceranemota partida Ceranemota albertae Ceranemota amplifascia Bycombia verdugoensis Euthyatira pudens Dogwood Thyatirid Drepana arcuata Arched Hooktip Drepana bilineata Two-lined Hooktip Eudeilinia herminiata Northern Eudeilinea Oreta rosea Rose Hooktip Eudeilinia luteifera Southern Eudeilinia Coloradia pandora Pandora pinemoth Coloradia luski Lusk's pinemoth Coloradia doris Doris' pinemoth Coloradia velda Velda pinemoth Automeris io Io moth Automeris louisiana Louisiana eyed silkmoth Automeris randa Randa's eyed silkmoth Automeris iris Iris eyed silkmoth Automeris cecrops Cecrops eyed silkmoth Automeris zephyria Zephyr eyed silkmoth Automeris patagoniensis Patagonia eyed silkmoth Hylesia coinopus Hemileuca juno Juno buckmoth Hemileuca maia Eastern buckmoth Hemileuca nevadensis Nevada buckmoth Hemileuca artemis Hemileuca lucina New England buckmoth Hemileuca slosseri Slosser's buckmoth Hemileuca peigleri Hemileuca grotei Grote's buckmoth Hemileuca stonei Stone's buckmoth Hemileuca electra Electra buckmoth Hemileuca tricolor Tricolor buckmoth Hemileuca hualapai Hualapai buckmoth Hemileuca oliviae Range caterpillar moth Hemileuca burnsi Burns' buckmoth Hemileuca -

Andrade-Campuzano Articulo.Pdf
APLICACIÓN DE LA TÉCNICA DEL CÓDIGO DE BARRAS DE LA VIDA PARA EVALUAR LA DIVERSIDAD DE ESPECIES EN LA FAMILIA CRAMBIDAE (LEPIDOPTERA) EN LA RESERVA GUADUALITO, QUINDÍO. Alfonso Andrade-Campuzano 1, Luisa Arcila-Cardona 2, Nicola S. Flanagan 3 y Rodrigo Bernal 4. 1. Pontificia Universidad Javeriana-Cali. Correo: [email protected] 2. Universidad del Valle. Correo: [email protected] 3. Pontificia Universidad Javeriana-Cali. Correo: [email protected] 4. Reserva Natural Guadualito. Correo: [email protected] Resumen: La pérdida de biodiversidad a nivel global es un gran reto en términos ambientales, e identificar esta biodiversidad para implementar estrategias de conservación eficaces es el desafío de la taxonomía moderna. El método de Código de Barras de la Vida (Barcoding), es una aproximación desarrollada para identificar especies de una manera rápida y precisa usando regiones genéticas cortas y estandarizadas. Los lepidópteros son un orden altamente diverso que presenta varios casos de cripticismo entre sus especies. La familia Crambidae es una familia de polillas altamente diversa y difícil de identificar morfológicamente, y frecuentemente son confundidas con especies de su familia hermana Pyralidae. Las especies de la familia Crambidae han sido estudiadas por ser plagas de cultivos, pero se presenta un gran vacío en cuanto a la riqueza de esta familia. En este estudio se realizó un inventario de la diversidad de las polillas Crambidae en la Reserva Guadualito, ubicada en la zona Andina colombiana. Se muestrearon 45 individuos, de los cuales 24 correspondieron a 20 especies distintas con un porcentaje de identidad genética mayor a 97%. Las otras 21 muestras se pudieron identificar sólo hasta el nivel de género o taxonomía superior. -

Ep 3111763 A1
(19) TZZ¥___¥_T (11) EP 3 111 763 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 04.01.2017 Bulletin 2017/01 A01N 43/58 (2006.01) A01N 43/653 (2006.01) A01P 7/00 (2006.01) (21) Application number: 15175092.4 (22) Date of filing: 02.07.2015 (84) Designated Contracting States: (71) Applicant: BASF Agro B.V. AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 6835 EA Arnhem (NL) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR (72) Inventor: Mazuir, Florent Designated Extension States: 68165 Mannheim (DE) BA ME Designated Validation States: (74) Representative: BASF IP Association MA BASF SE ZRX-C6 67056 Ludwigshafen (DE) (54) PESTICIDAL COMPOSITIONS COMPRISING A TRIAZOLE COMPOUND (57) The present invention relates to pesticidal compositions and uses and methods for combatting harmful pests using the inventive compositions, such as phythopathogenic fungi and/or for the control of insects, acarids or nematodes. Furthermore, the present invention provides methods for improving the health of a plant using the inventive compositions. EP 3 111 763 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 111 763 A1 Description [0001] The present invention relates to pesticidal compositions and uses and methods for combatting harmful pests using the inventive compositions, such as phythopathogenic fungi and/or for the control of insects, acarids or nematodes. 5 Furthermore, the present invention provides methods for improving the health of a plant using the inventive compositions. -

Notes on Lepidoptera from the Seychelles
Phelsuma 24 (2016); 35-71 Notes on Lepidoptera from the Seychelles Maik Bippus 193 bis CD 41, 97419 La Possession, LA RÉUNION [[email protected]] Abstract: 53 species of Lepidoptera are reported from Mahé and Praslin, 44 species are illustrated. Genitalia images for 25 species are provided. Key words: Lepidoptera, Seychelles In July 2014 I spent a short holiday on the inner islands of the Seychelles (Mahé & Praslin) and took the opportunity to track some Lepidoptera. Apparently some islands, notably Praslin, had only scarcely been researched in the past. For Praslin 54 species of Lepidoptera are presently known although only very recently 11 species had been added by Bolotov et al. (2015). In this paper, I would like to share my results with the scientific community and I hope that this will incite other naturalists to undertake more research on the islands. For Praslin I can add another 31 species of Heterocera, 3 new recorded species for the Granitic Islands of the Seychelles and I would like to point out to 3 possible new synonymes that will need additional verifications. I regret that I cannot myself examine the types of the different suggested synonyms for geographical reasons and I would like to invite other researchers to take a closer look at them at their next revision of the respective genera or families. Stations: all Lepidoptera caught in Praslin were collected at Anse Boudin, at the junction of the main road to Zimbabwe at an altitude of 25m ��������������������������or the nearby surroundings (4�17�55��’’�����S, 55�42�35’’E���). -
Occurrence of Genus Amyna (Noctuidae: Bagisarinae) Among
0Journal of Entomology and Zoology Studies 2017; 5(1): 949-953 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Occurrence of genus Amyna (Noctuidae: JEZS 2017; 5(1): 949-953 © 2017 JEZS Bagisarinae) among the semilooper complex Received: 07-11-2016 Accepted: 08-12-2016 infesting soybean at Udaipur Ashok Kumar Meena Department of Entomology, Rajasthan College of Agriculture, Ashok Kumar Meena, Rajendra Nagar and R Swaminathan Maharana Pratap University of Agriculture and Technology, Abstract Udaipur, Rajasthan, India The semilooper complex infesting soybean was studied in a field experiment laid out at the instructional farm of Rajasthan College of Agriculture, Maharana Pratap University of Agriculture and Technology, Rajendra Nagar Department of Entomology, Udaipur, during kharif 2016. The semilooper complex included the following species: Trichoplusia ni Rajasthan College of Agriculture, (Hubner), Chrysodeixis includens (Walker), Chrysodeixis acuta (Walker), Chrysodeixis chalcites Maharana Pratap University of (Esper), Amyna axis (Guenee) and Amyna natalis (Walker). The semilooper population was the Agriculture and Technology, maximum during mid-August (7.25/5 plants) and evinced a significant negative correlation with the Udaipur, Rajasthan, India mean atmospheric temperature (r = - 0.64); while, a significant positive correlation with the mean relative humidity (r= + 0.86) and total rainfall (r= + 0.61), notable being abundant after the rains. The R Swaminathan morphological characterization of the two species of Amyna is given with suitable photographs, -
Lepidoptera, Noctuidae)
A peer-reviewed open-access journal ZooKeys Larva 39: 107–116 and pupa (2010) of Amyna axis (Guenée, 1852) and affi rmation of its taxonomic placement... 107 doi: 10.3897/zookeys.39.435 RESEARCH ARTICLE www.pensoftonline.net/zookeys Launched to accelerate biodiversity research Larva and pupa of Amyna axis (Guenée, 1852) and affirmation of its taxonomic placement in Bagisarinae (Lepidoptera, Noctuidae) David L. Wagner, Shawn Binns Department of Ecology and Evolutionary Biology, Univ. Box 43, University of Connecticut, Storrs, Connecticut 06269 Corresponding author: David L. Wagner ([email protected]) Academic editor: J. D. Lafontaine | Received 15 January 2010 | Accepted 17 February 2010 | Published 18 March 2010 Citation: Wagner DL, Binns S (2010) Larva and pupa of Amyna axis (Guenée, 1852) and affi rmation of its taxonomic placement in Bagisarinae (Lepidoptera, Noctuidae). In: Schmidt BC, Lafontaine JD (Eds) Contributions to the systema- tics of New World macro-moths II. ZooKeys 39: 107–116. doi: 10.3897/zookeys.39.435 Abstract Th e larva and pupa of Amyna axis (Guenée, 1852) are described and illustrated, and observations are provided on the insect’s life history and larval biology. Larval, adult, and life history characters support the transfer of Amyna Guenée from Acontiinae Guenée, 1841 to Bagisarinae Crumb, 1956. Th e phylogenetic placement of the Bagisarinae is enigmatic; some adult and larval features indicate that the subfamily is a basal trifi d proximate to Acontiinae, whereas other larval and life history characters suggest an associa- tion with Scoliopteryginae, a basal quadrifi d group. Larvae exhibit a green-to-black color polyphenism presumably linked to larval density, with darker phenotypes occurring during outbreak densities. -

Rapid Biodiversity Assessment of Key Biodiversity Areas: Falealupo Peninsula Coastal Rainforest, Central Savai'i Rainforest, A
Rapid Biodiversity Assessment of Key Biodiversity Areas: Falealupo Peninsula Coastal Rainforest, Central Savai’i Rainforest, and Uafato-Tiavea Coastal Rainforest, Samoa March 2017 ISBN: © 2017 Ministry of Natural Resources and Environment, Conservation International Pacific Islands Programme. Suggested citation: Ministry of Natural Resources and Environment, Conservation International Pacific Islands Programme. 2017. Rapid Biodiversity Assessment of Key Biodiversity Areas: Falealupo Peninsula Coastal Rainforest, Central Savaii Rainforest, and Uafato-Tiavea Coastal Rainforest, Samoa. Apia, Samoa. 285pp. Cover photos: Top left: Taga montane rainforest (Mark O’Brien) Top right: Micronesian skink (Jonathan Richmond) Bottom Left: Thalassodes species emerald moth (Eric Edwards) Bottom Right: Samoan Broadbill (James Atherton) i Table of Contents Authors ................................................................................................................................................ vi Organizational Profiles ........................................................................................................................ vii Acknowledgements ............................................................................................................................... x Acronyms ............................................................................................................................................. xi Foreword ........................................................................................................................................... -

Performance of Amaranth Accessions Against Moisture Stress and Key Insect Pests and Their Indigenous Parasitoids in Arusha, Tanzania
PERFORMANCE OF AMARANTH ACCESSIONS AGAINST MOISTURE STRESS AND KEY INSECT PESTS AND THEIR INDIGENOUS PARASITOIDS IN ARUSHA, TANZANIA STEPHEN TARMOGIN OMBURO OTHIM (MSc. Crop Prot. Ento) A99/38397/2016 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE AWARD OF DOCTOR OF PHILOSOPHY IN AGRICULTURAL ENTOMOLOGY OF KENYATTA UNIVERSITY. NOVEMBER 2019 DECLARATION I Stephen Tarmogin Omburo Othim declare that this thesis is my original work and has not been presented for the award of a degree in any other university or any other award. Signature …………………………Date…………………………………. Declaration by supervisors We confirm that the work reported in this thesis was carried out by the candidate under our supervision and has been submitted with our approval as supervisors. Signature………................................Date …………………………… Dr. Ruth Kahuthia-Gathu Department of Agricultural Science and Technology Kenyatta University (KU) Signature………................................Date ……………………………… Dr. Komi Kouma Mokpokpo Fiaboe Plant Health Theme International Centre of Insect Physiology and Ecology (icipe) Signature ............................................Date …………………………… Dr. Srinivasan Ramasamy Entomology department World Vegetable Centre (WorldVeg) ii DEDICATION Dedicated to my loving and dearest wife Diana Nyanting’a who has been my greatest source of inspiration and whose tolerance and sacrifice has seen me complete this thesis. iii ACKNOWLEDGEMENTS Greatest appreciation to the Almighty God who Has been faithful and brought me to this great achievement. I extend my deepest appreciation to my loving wife Diana Nyanting’a for her endless motivation and moral support during my research. I sincerely thank my supervisors Dr. Ruth Kahuthia-Gathu of Kenyatta University (KU), Dr. Komi Fiaboe of International Centre of Insect Physiology and Ecology (icipe)/International Institute of Tropical Agriculture (IITA) and Dr. -
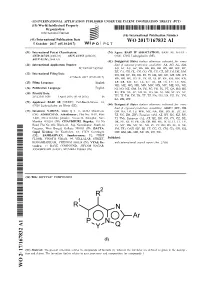
WO 2017/167832 Al 5 October 2017 (05.10.2017) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/167832 Al 5 October 2017 (05.10.2017) P O P C T (51) International Patent Classification: (74) Agent: BASF IP ASSOCIATION; BASF SE, G-FLP - C07D 487/04 (2006.01) A01N 43/653 (2006.01) C006, 67056 Ludwigshafen (DE). A01N 43/54 (2006.01) (81) Designated States (unless otherwise indicated, for every (21) International Application Number: kind of national protection available): AE, AG, AL, AM, PCT/EP2017/057464 AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, (22) Date: International Filing DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, 29 March 2017 (29.03.2017) HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KH, KN, (25) Filing Language: English KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, (26) Publication Language: English NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, (30) Priority Data: RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, 20162101 1658 1 April 2016 (01.04.2016) IN TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (71) Applicant: BASF SE [DE/DE]; Carl-Bosch-Strasse 38, 67056 Ludwigshafen am Rhein (DE). (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (72) Inventors: NARINE, Arun; Q 3, 12, 6816 1 Mannheim GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, (DE).