Abundance and Spatial Distribution of Mosquitoes Across Three Ecological
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
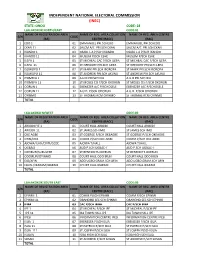
Ondo Code: 28 Lga:Akokok North/East Code:01 Name of Registration Area Name of Reg
INDEPENDENT NATIONAL ELECTORAL COMMISSION (INEC) STATE: ONDO CODE: 28 LGA:AKOKOK NORTH/EAST CODE:01 NAME OF REGISTRATION AREA NAME OF REG. AREA COLLATION NAME OF REG. AREA CENTRE S/N CODE (RA) CENTRE (RACC) (RAC) 1 EDO 1 01 EMMANUEL PRI.SCHEDO EMMANUEL PRI.SCHEDO 2 EKAN 11 02 SALEM A/C PRI.SCH EKAN SALEM A/C PRI.SCH EKAN 3 IKANDO 1 03 OSABL L.A P/SCH IKANDO OSABL L.A P/SCH IKANDO 4 IKANDO 11 04 MUSLIM P/SCH ESHE MUSLIM P/SCH ESHE 5 ILEPA 1 05 ST MICHEAL CAC P/SCH ILEPA ST MICHEAL CAC P/SCH ILEPA 6 ILEPA 11 06 ST GREGORY PRI.SCH ILEPA ST GREGORY PRI.SCH ILEPA 7 ISOWOPO 1 07 ST MARK PRI.SCH IBOROPA ST MARK PRI.SCH IBOROPA 8 ISOWOPO 11 08 ST ANDREW PRI.SCH AKUNU ST ANDREW PRI.SCH AKUNU 9 IYOMEFA 1 09 A.U.D PRI.SCH IKU A.U.D PRI.SCH IKU 10 IYOMEFA 11 10 ST MOSES CIS P/SCH OKORUN ST MOSES CIS P/SCH OKORUN 11 OORUN 1 11 EBENEZER A/C P/SCHOSELE EBENEZER A/C P/SCHOSELE 12 OORUN 11 12 A.U.D. P/SCH ODORUN A.U.D. P/SCH ODORUN 13 OYINMO 13 ST THOMAS RCM OYINMO ST THOMAS RCM OYINMO TOTAL LGA:AKOKO N/WEST CODE:02 NAME OF REGISTRATION AREA NAME OF REG. AREA COLLATION NAME OF REG. AREA CENTRE S/N CODE (RA) CENTRE (RACC) (RAC) 1 ARIGIDI IYE 1 01 COURT HALL ARIGIDI COURT HALL ARIGIDI 2 ARIGIDI 11 02 ST JAMES SCH IMO ST JAMES SCH IMO 3 OKE AGBE 03 ST GOERGE P/SCH OKEAGBE ST GOERGE P/SCH OKEAGBE 4 OYIN/OGE 04 COMM.P/SCH OKE AGBE COMM.P/SCH OKE AGBE 5 AJOWA/ILASI/ERITI/GEDE 05 AJOWA T/HALL AJOWA T/HALL 6 OGBAGI 06 AUD P.SCH OGBAC-I AUD P.SCH OGBAC-I 7 OKEIRUN/SURULERE 07 ST BENEDICTS OKERUN ST BENEDICTS OKERUN 8 ODOIRUN/OYINMO 08 COURT HALL ODO IRUN COURT HALL ODO IRUN 9 ESE/AFIN 09 ADO UGBO GRAM.SCH AFIN ADO UGBO GRAM.SCH AFIN 10 EBUSU/IKARAM/IBARAM 10 COURT HALL IKARAM COURT HALL IKARAM TOTAL LGA:AKOKOK SOUTH EAST CODE:03 NAME OF REGISTRATION AREA NAME OF REG. -

Nigeria's Constitution of 1999
PDF generated: 26 Aug 2021, 16:42 constituteproject.org Nigeria's Constitution of 1999 This complete constitution has been generated from excerpts of texts from the repository of the Comparative Constitutions Project, and distributed on constituteproject.org. constituteproject.org PDF generated: 26 Aug 2021, 16:42 Table of contents Preamble . 5 Chapter I: General Provisions . 5 Part I: Federal Republic of Nigeria . 5 Part II: Powers of the Federal Republic of Nigeria . 6 Chapter II: Fundamental Objectives and Directive Principles of State Policy . 13 Chapter III: Citizenship . 17 Chapter IV: Fundamental Rights . 20 Chapter V: The Legislature . 28 Part I: National Assembly . 28 A. Composition and Staff of National Assembly . 28 B. Procedure for Summoning and Dissolution of National Assembly . 29 C. Qualifications for Membership of National Assembly and Right of Attendance . 32 D. Elections to National Assembly . 35 E. Powers and Control over Public Funds . 36 Part II: House of Assembly of a State . 40 A. Composition and Staff of House of Assembly . 40 B. Procedure for Summoning and Dissolution of House of Assembly . 41 C. Qualification for Membership of House of Assembly and Right of Attendance . 43 D. Elections to a House of Assembly . 45 E. Powers and Control over Public Funds . 47 Chapter VI: The Executive . 50 Part I: Federal Executive . 50 A. The President of the Federation . 50 B. Establishment of Certain Federal Executive Bodies . 58 C. Public Revenue . 61 D. The Public Service of the Federation . 63 Part II: State Executive . 65 A. Governor of a State . 65 B. Establishment of Certain State Executive Bodies . -

Gender Sensitivity to Public School Enrolment in Ondo State, Nigeria Using Systematic Sampling
DOI: http://dx.doi.org/10.4314/gjpas.v22i1.8 GLOBAL JOURNAL OF PURE AND APPLIED SCIENCES VOL. 22, 2016: 61-71 COPYRIGHT© BACHUDO SCIENCE CO. LTD PRINTED IN NIGERIA ISSN 1118-0579 61 www.globaljournalseries.com, Email: [email protected] GENDER SENSITIVITY TO PUBLIC SCHOOL ENROLMENT IN ONDO STATE, NIGERIA USING SYSTEMATIC SAMPLING ADEDAYO AMOS ADEWARA AND ADEBOLA ANTHONIA ADENIYI (Received 15 December 2015; Revision Accepted 3 March 2016) ABSTRACT This study employed the total number of public schools in Ondo, Nigeria which gives information on the name of the school, males and females in each school from JSS1 to SS3 in the eighteen local government areas. Systematic sampling was used to select forty eight schools from the two hundred and eighty eight public schools in the state during the 2012/2013 and 2013/2014 academic sections. Applying the instrument of Pearson product moment correlation coefficient, the study revealed that (i).females are more sensitive to public school enrolment during 2012/2013 and 2013/2014 academic sessions than their males counterparts, (ii).there is significant difference between the female enrolments during the two academic sessions, (iii).there is significant difference between the male enrolments during the two academic sessions and (iv). the trend of male female enrolments in the selected public schools shows improvement during 2013/2014 over that of 2012/2013 academic session. KEYWORD: Gender, Sensitivity, Systematic Sampling, School, Enrolment INTRODUCTION University of Technology, Akure, Adekunle Ajasin University, Akungba/Akoko, Ondo State polytechnic, The foundation of any state is in the number of Owo, Federal College of Agriculture, Akure and people who are literate in that particular state. -

The Case Study of Owo LGA, Ondo State, Nigeria
The International Journal Of Engineering And Science (IJES) ||Volume||2 ||Issue|| 9 ||Pages|| 19-31||2013|| ISSN(e): 2319 – 1813 ISSN(p): 2319 – 1805 Geo-Information for Urban Waste Disposal and Management: The Case Study of Owo LGA, Ondo State, Nigeria *1Dr. Michael Ajide Oyinloye and 2Modebola-Fadimine Funmilayo Tokunbo Department of Urban and Regional Planning, School of Environmental Technology, Federal University of Technology, Akure, Nigeria --------------------------------------------------------ABSTRACT-------------------------------------------------- Management of waste is a global environmental issue that requires special attention for the maintenance of quality environment. It has been observed that amount, size, nature and complexity of waste generated by man are profoundly influenced by the level of urbanization and intensity of socio-economical development in a given settlement. The problem associated with its management ranges from waste generation, collection, transportation, treatment and disposal. The study involves a kind of multi-criteria evaluation method by using geographical information technology as a practical instrument to determine the most suitable sites of landfill location in Owo Local Government Area of Ondo state. Landsat Enhanced Thematic Mapper plus (ETM+) 2002 and updated 2012 were used to map the most suitable site for waste disposal in Owo LGA. The result indicates that sites were found within the study area. The most suitable sites in the study area are located at 200metre buffer to surface water and 100metre to major and minor roads. The selected areas have 2500metres buffer zone distance from urban areas (built up areas). The study purposes acceptable landfill sites for solid waste disposal in the study area. The results achieved in this study will help policy and decision makers to take appropriate decision in considering sanitary landfill sites. -

Geoelectric Sounding to Delineate Shallow Aquifers in the Coastal Plain Sands of Okitipupa Area, Southwestern Nigeria
Geoelectric Sounding to Delineate Shallow Aquifers in the Coastal Plain Sands of Okitipupa Area, Southwestern Nigeria. G.O. Omosuyi, J.S. Ojo, and M.O. Olorunfemi* Department of Applied Geophysics, Federal University of Technology, PMB 704, Akure, Ondo State, Nigeria. *Department of Geology, Obafemi Awolowo University, Ile-Ife, Nigeria. ABSTRACT INTRODUCTION In Okitipupa area, south western Nigeria, the The Okitipupa area is underlain by the aquifers of the Coastal Plain sands are largely sedimentary rocks of south western Nigeria. The Quaternary porous sediments, but without records zone constitutes the easternmost segment of the of their subsurface disposition. In this study, fifty extensive Dahomey basin. Studies have shown Schlumberger soundings were conducted over the that sedimentary basins generally contain study area, aimed at delineating the boundaries, enormous quantities of water (Freeze and depth range of the shallow aquifer units, and Cherry, 1979; Fetter, 1980; Kehinde and assessing their vulnerability to near-surface Loehnert, 1989; Edet and Okereke, 2002). To contaminants. realise this fact, several countries worldwide have engaged in extensive studies of basins within The study delineated two major aquifer units their frontiers, using geological/hydrogeological, within the area: (i) the upper/surficial aquifer geophysical, or chemical data, or a combination system, which occur at depths ranging from 5.8m of the above, for proper identification and (around Agbabu) to 61.5m (around Ikoya), and delineation of the water bearing -

Ondo State Pocket Factfinder
INTRODUCTION Ondo is one of the six states that make up the South West geopolitical zone of Nigeria. It has interstate boundaries with Ekiti and Kogi states to the north, Edo State to the east, Delta State to the southeast, Osun State to the northwest and Ogun State to the southwest. The Gulf of Guinea lies to its south and its capital is Akure. ONDO: THE FACTS AND FIGURES Dubbed as the Sunshine State, Ondo, which was created on February 3, 1976, has a population of 3,441,024 persons (2006 census) distributed across 18 local councils within a land area of 14,788.723 Square Kilometres. Located in the South-West geo-political zone of Nigeria, Ondo State is bounded in the North by Ekiti/Kogi states; in the East by Edo State; in the West by Oyo and Ogun states, and in the South by the Atlantic Ocean. Ondo State is a multi-ethnic state with the majority being Yoruba while there are also the Ikale, Ilaje, Arogbo and Akpoi, who are of Ijaw extraction and inhabit the riverine areas of the state. The state plays host to 880 public primary schools, and 190 public secondary schools and a number of tertiary institutions including the Federal University of Technology, Akure, Ondo State Polytechnic, Owo, Federal College of Agriculture, Akure and Adeyemi College of Education, Ondo. Arguably, Ondo is one of the most resource-endowed states of Nigeria. It is agriculturally rich with agriculture contributing about 75 per cent of its Gross Domestic Product (GDP). The main revenue yielding crops are cocoa, palm produce and timber. -

Ondo State Conflictbulletin: Nigeria
THE FUND FOR PEACE Nigeria Conflict Bulletin: Ondo State Patterns and Trends, January 2012 - J u n e 2 0 1 5 While violence in Ondo has historically Democratic Party (PDP). The next page shows the relative distribution of been relatively low, in the first half of 2015 gubernatorial elections are scheduled for incidents from one LGA to the next from reported fatalities increased significantly as 2016. January 2012 to June 2015. The trendline on compared to previous years. This was the next page shows the number of mainly in connection to a few incidents of This Conflict Bulletin provides a brief incidents and fatalities over time. The bar criminality (bank robberies in Owo and snapshot of the trends and patterns of chart shows the relative trend of incidents Akoko North West LGAs) and piracy (Ilaje conflict risk factors at the State and LGA of insecurity by LGA per capita. LGA) that killed dozens. Other issues, levels, drawing on the data available on the reported in Ondo included political tensions P4P Digital Platform for Multi-Stakeholder The summaries draw on data collected by and cult violence. Engagement (www.p4p-nigerdelta.org). It ACLED, FFP’s UNLocK, the Council on represents a compilation of the data from Foreign Relations’ NST, WANEP Nigeria, CSS/ After the 2012 gubernatorial election, in the sources listed below, not necessarily the ETH Zurich, NEEWS2015, and Nigeria Watch which Olusegun Mimiko of the Labour Party opinions of FFP or any other organization integrated on the P4P platform. They also (LP) was re-elected, the losing parties raised that collaborated on the production of this draw on data and information from concerns about alleged election bulletin. -

States and Lcdas Codes.Cdr
PFA CODES 28 UKANEFUN KPK AK 6 CHIBOK CBK BO 8 ETSAKO-EAST AGD ED 20 ONUIMO KWE IM 32 RIMIN-GADO RMG KN KWARA 9 IJEBU-NORTH JGB OG 30 OYO-EAST YYY OY YOBE 1 Stanbic IBTC Pension Managers Limited 0021 29 URU OFFONG ORUKO UFG AK 7 DAMBOA DAM BO 9 ETSAKO-WEST AUC ED 21 ORLU RLU IM 33 ROGO RGG KN S/N LGA NAME LGA STATE 10 IJEBU-NORTH-EAST JNE OG 31 SAKI-EAST GMD OY S/N LGA NAME LGA STATE 2 Premium Pension Limited 0022 30 URUAN DUU AK 8 DIKWA DKW BO 10 IGUEBEN GUE ED 22 ORSU AWT IM 34 SHANONO SNN KN CODE CODE 11 IJEBU-ODE JBD OG 32 SAKI-WEST SHK OY CODE CODE 3 Leadway Pensure PFA Limited 0023 31 UYO UYY AK 9 GUBIO GUB BO 11 IKPOBA-OKHA DGE ED 23 ORU-EAST MMA IM 35 SUMAILA SML KN 1 ASA AFN KW 12 IKENNE KNN OG 33 SURULERE RSD OY 1 BADE GSH YB 4 Sigma Pensions Limited 0024 10 GUZAMALA GZM BO 12 OREDO BEN ED 24 ORU-WEST NGB IM 36 TAKAI TAK KN 2 BARUTEN KSB KW 13 IMEKO-AFON MEK OG 2 BOSARI DPH YB 5 Pensions Alliance Limited 0025 ANAMBRA 11 GWOZA GZA BO 13 ORHIONMWON ABD ED 25 OWERRI-MUNICIPAL WER IM 37 TARAUNI TRN KN 3 EDU LAF KW 14 IPOKIA PKA OG PLATEAU 3 DAMATURU DTR YB 6 ARM Pension Managers Limited 0026 S/N LGA NAME LGA STATE 12 HAWUL HWL BO 14 OVIA-NORTH-EAST AKA ED 26 26 OWERRI-NORTH RRT IM 38 TOFA TEA KN 4 EKITI ARP KW 15 OBAFEMI OWODE WDE OG S/N LGA NAME LGA STATE 4 FIKA FKA YB 7 Trustfund Pensions Plc 0028 CODE CODE 13 JERE JRE BO 15 OVIA-SOUTH-WEST GBZ ED 27 27 OWERRI-WEST UMG IM 39 TSANYAWA TYW KN 5 IFELODUN SHA KW 16 ODEDAH DED OG CODE CODE 5 FUNE FUN YB 8 First Guarantee Pension Limited 0029 1 AGUATA AGU AN 14 KAGA KGG BO 16 OWAN-EAST -

Computation of Water-Stress Ratio in Western Nigeria by P
Global Journal of Science Frontier Research Agriculture and Veterinary Sciences Volume 12 Issue 7 Version 1.0 Year 2012 Type : Double Blind Peer Reviewed International Research Journal Publisher: Global Journals Inc. (USA) Online ISSN: 2249-4626 & Print ISSN: 0975-5896 Computation of Water-Stress Ratio in Western Nigeria By P. O Idogho & Olotu Yahaya Auchi Polytechnic, Auchi Abstract - An increasing world population exerts a continually growing demand on usable freshwater resources. Access to water plays a key role in development; it supports human life in direct consumption, agricultural uses and industrial activities. Time and drudgery involved to access safe drinking water resulted to loss of human resources and capital, thus affecting nearly every household life. This paper focuses on the determination of water-stressed ratio using Integrated Water Measurement Tool (IWMT). Structured simple time analysis and Adjusted composite index approaches were employed to compute (IWMT) values in all the sampled areas. Variables such as access to safe water coverage, water availability and use of water were considered. IWMT values from the two approaches show that Ese-Odo is the most water-scarce region with least IWMT values of 14.1 (Adjusted composite index: ACI) and highest value of 2.6 minsl-1 (Structured simple time analysis: SSA), while Owo, Ondo-West and Ose local government areas experience fair distribution of protected water supply with IWMT values of 1.05 minsl-1, 20.8; 1.00 minsl-1, 17.2; and 0.55 minsl-1, 16.9 respectively. Keywords : IWMT, index, water stress, safe water, access, water-scarce, uses, household, ACI, SSA, ratio. -

Peace and War in Ilaje-Ugbo/Arogbo-Ijaw Relations from the Pre-Colonial Era up to 1999
International Journal of Research and Innovation in Social Science (IJRISS) |Volume IV, Issue IV, April 2020|ISSN 2454-6186 Peace and War in Ilaje-Ugbo/Arogbo-Ijaw Relations from the Pre-Colonial Era Up to 1999 Oluwafunke Adeola ADELEYE1, David Olanrewaju OGORU2, Johnson Olaosebikan AREMU (PhD)3 1,2,3Department of History and International Studies, Ekiti State University, Ado Ekiti, Nigeria Abstract: - This paper examined the twin factors of peace and each other in a violent war that brought deaths, destruction of war that have defined inter-group relations between the Arogbo- property and significant number of internally displaced Ijaw and Ilaje-Ugbo communities in present-day Ondo State, persons in its trail. It is in view of this that this paper Nigeria. The specific objectives of the study are to: identify the examines the factors of peace and war in the volatile relations elements of intergroup relations between the two communities; between the two groups. The paper is divided into six sections analyse the dynamic nature of relations between the two peoples; highlight the remote and immediate causes of conflict and war in viz: introduction, meaning of inter-group relations, elements their relations and; suggest ways of restoring harmony and of peaceful relations, the 1998-1999 Ilaje/Ijaw war, with peaceful co-existence between the two ethic groups. Data for the emphasis on its causes and effects; and conclusion. study was generated through primary and secondary sources. II. DYNAMICS OF ILAJE-UGBO/AROGBO-IJAW The primary sources include archival materials, private diaries of some community leaders as well as unstructured interviews RELATIONS FROM THE EARLIEST TIMES TO 1999 conducted with some informants. -

BRIEF HISTORY Ondo State Was Created in 1976 out of the Former
9 Ijebu North 82 10 Ijebu North East 94 11 Ijebu Ode 70 12 Ikenne 50 13 Imeko / Afon 80 14 Ipokia 120 15 Obafemi / Owode 20 16 Odeda 30 17 Odogbolu 58 18 0gun Waterside 201 19 Remo North 58 20 Sagamu 45 ONDO BRIEF HISTORY Ondo State was created in 1976 out of the former Western State. The state lies between latitudes 5 45’ and 7 52’N and longitudes 4 20’ and 6 03’E. It is bounded on the east by Edo and Delta states, on the west by Ogun and Osun states, on the north by Ekiti and Kogi states, and to the south by the Bight of Benin of the Atlantic Ocean. The state occupies a land area of about 15,000 Square kilometers with a population of 3,441,924 people according to 2006 census. The state has eighteen (18) Local Government Areas, with Akure as the capital city as well as the largest settlement. Some of the other prominent towns in the state are Ondo, Owo, Ore, Okitipupa, Ikare, Idanre, and Ile-Oluji. The people of the state are mostly Yoruba, although other Nigerians and foreign nationals equally live in the state. Agriculture is the mainstay of the people of Ondo State. The state has notable tourist attractions including Owo Museum, Ipale Iloro Water Falls, Igbokoda Water Front, Oke Maria Hills and Olumirin Water Falls. SENATORIAL DISTRICTS S/N DISTRICT LGA COMPOSITION RA PU COLLATION CENTRE 82 Ondo North Akoko North East/ Akoko North 72 889 Mapo Hall Owo SD/082/0D West/Akoko South East/Akoko South West/Owo Ose 83 Ondo Akure North/Akure South/Idanre 65 978 St. -

ISSN: 2320-5407 Int
ISSN: 2320-5407 Int. J. Adv. Res. 6(2), 397-407 Journal Homepage: -www.journalijar.com Article DOI:10.21474/IJAR01/6451 DOI URL: http://dx.doi.org/10.21474/IJAR01/6451 RESEARCH ARTICLE GEOGRAPHICAL ANALYSIS OF ONDO STATE GUBERNATORIAL ELECTION RESULTS OF 2012 AND 2016. Richard Babajide Adeleke. Department of Geography, University of Ibadan. …………………………………………………………………………………………………….... Manuscript Info Abstract ……………………. ……………………………………………………………… Manuscript History Space is one of the key drivers of election results and the few studies on the influence of space on election results in Nigeria reported a mere Received: 05 December 2017 spatial distribution without giving an insight into their spatial Final Accepted: 07 January 2018 clustering. Using Global and Local Moran’s I geospatial techniques, the Published: February 2018 study found a significant positive spatial autocorrelation and clustering Keywords:- of votes in 2012 election for the candidates of Peoples Democratic Election, GIS, Spatial Autocorrelation, Party in Ilaje, Irele and Ese-Odo local government areas while that of Ondo State, Nigeria Action Congress of Nigeria was in Owo and Akoko Southwest. In 2016 election, that of All Progressives Congress was in Owo and Ondo East. However, there was a significant negative spatial autocorrelation and clustering of votes for the candidates of Labour Party in Akure North and Alliance for Democracy in Ese-Odo and Ilaje in 2012 election. On the whole, the pattern of votes cast for the victorious party in 2016 election was found to be more regionally concentrated when compared to 2012. The study concluded that a lot still has to be done with regard to voters’ education. Copy Right, IJAR, 2018,.