Extraction of Uranium (Vi) from Nitric Acid and Nitrate Solutions by Tributylphosphate/Kerosene
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Extraction Processing of Concentrated Solutions of Uranyl Nitrate with High Impurities Content V.M
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Electronic archive of Tomsk Polytechnic University Сhemistry UDC 546.791.02.238:66.061.51 EXTRACTION PROCESSING OF CONCENTRATED SOLUTIONS OF URANYL NITRATE WITH HIGH IMPURITIES CONTENT V.M. Korotkevich, V.V.Lazarchuck, T.G. Shikerun, V.I. Shamin, N.A. Mikhailova, F.A. Dorda Federal Unitary Enterprise Siberian Group of Chemical Enterprises, Seversk Еmail: [email protected] Process flowsheet of recycling uranium concentrated solutions with its purification from insoluble impurities of iron, silicon, molybde num, calcium oxides and hydroxides and soluble impurities with application of centrifugal extractors cascade has been developed and suggested for commercial introduction. The process was carried out at extractant saturation (30 % tributyl phosphate in hydrocarbon diluent) in extraction assembly lower than a limiting level (85...95 g/l) and in wash assembly – at limiting saturation (up to 120 g/l). As a result the waste uranium content in watertail solutions 0,01...0,04 g/l and minimal content of impurities in reextractors is provided. Perspective program on development of nuclear technology are also: reliability of hermetization, possi power engineering proposes to involve wide range of raw bility of remote service, resistance to corrosive attack of materials from uranium ores to secondary uranium ma structural materials, duration of overhaul period etc. terials into processing at atomic enterprises. In this case In Russia the centrifugal extractor of ECТseries the problem of reprocessing concentrated uranium so with continuous extraction of solid phase [3], which may lutions with high impurity content occurs. -
Nitrate (UO2 (NO)) 4
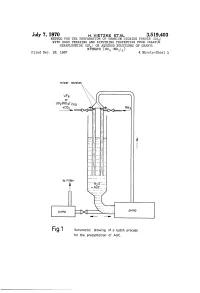
July 7, 1970 H. VETZKE ETA 3,519,403 METHOD FOR THE PREPARATION OF URANIUM DIOXIDE POWDER (UO) WITH GOOD PRESSING AND SINTERING PROPERTIES FROM URANIUM HEXAFLUORIDE (UF) OR AQUEOUS SOLUTIONS OF URANYL Fied Dec. 18, 1967 NiTRATE (UO2 (NO)) 4. Sheets-Sheet l mixer nozzles --race to fitter Fig.1 Schematic drawing of a batch process for the precipitation of AUC. July 7, 1970 H. VETZKE EA 3,519,403 METHOD FOR THE PREPARATION OF URANIUM DIOXIDE POWDER (UO) WITH GOOD PRESSING AND SINTERING PROPERTIES FROM RANIUM HEXAFLUORIDE (UF) OR AQUEOUS SOLUTIONS OF URANYL Filed Dec. 8, 1967 NiTRATE (UO2 (NO)) 4. Sheets-Sheet 3. mixer nozzles UF6 Of U02 (NO3)2ad tC92--X Ex-Ns / to filter cooling precipitation Vessel vessel Fig. 2 Schematic drawing of a continuous process for the precipitation of AUC July 7, 1970 H, VETZKE ETAL 3,519,403 METHOD FOR THE PREPARATION OF URANIUM DIOXIDE POWDER (UO) WITH GOOD PRESSING AND SINTERING PROPERTIES FROM URANIUM HEXAFLUORIDE (UF) OR AQUEOUS SOLUTIONS OF URANYL Filed Dec. 18, 1967 NiTRATE (UO (NO)2) 4. Sheets-Sheet 3 Off gas Off gas 487\m Nx UO2 powder XXX.& X.X S& steam / H2 Fig. 3 Ot Schematic drawing of a fluidized bed furnace for the reduction of AUC to U02, July 7, 1970 H. VEZKE ETAL 3,519,403 METHOD FOR THE PREPARATION OF URANIUM DIOXIDE POWDER (UO) WITH GOOD PRESSING AND SINTERING PROPERTIES FROM URANIUM HEXAFLUORIDE (UF) OR AQUEOUS SOLUTIONS OF URANYL NITRATE (UO2 (NO)a) Filed Dec. 18, 1967 4. Sheets-Sheet 1 -- Offgas Step 1: Decomposition, reduction and pyrohydrolysis i Step 2: Pyrohydrolysis Step 3: Controlled oxidation an o- are as an un- Fig. -

Chemical Resistance List
Chemical Resistance List Resistance Substance Permeation Time/Level to Degradation Fluoro- natural chloro- nitrile/ nitrile carbon butyl latex prene chloroprene rubber NR CR CR NBR FKM IIR NR NR CR CR NBR NBR NBR NBR NBR FKM IIR IIR NBR NBR 395 450, 451 720, 722 717 727 730, 732 740, 741 743 754 764 890 897 898 chemical physical 403 706 723, 725 733, 836 742, 757 state 708 726 736 - 739 759 - 0 - 0 0 + 1-methoxy-2-propanol paste 4 2 2 3 4 4 B 1 3 4 6 6 - 0 - 0 0 + 1-methoxy-2-propyl acetate liquid 3 1 1 3 3 A B 2 3 6 6 - 0 0 - 0 + 1-methyl-2-pyrrolidone liquid 5 2 3 3 3 2 A B 1 3 3 6 6 - 0 + + + - 1,1,2-trichlorotrifluoroethane liquid 1 0 5 4 6 6 1 1 2 1 6 1 2 - - - - - - 1.2-epoxy ethane (ethylene oxide) liquid B A A A A 0 0 0 B 1 2 - - - - - - 1.2-epoxy propane (propylene oxide) liquid B A A A 1 A 0 0 0 B 1 2 + + + + + + 1.2-propanediol liquid 6 6 6 6 6 6 6 6 6 6 6 6 6 - + - + + 0 2-ethyl hexyl acrylate liquid 2 1 1 5 6 1 1 2 6 2 3 - 0 0 0 + + 2-mercaptoethanol liquid 3 2 4 4 4 4 1 1 3 6 6 6 - - - 0 0 - 2-methoxy-2-methyl propane liquid 1 B B 2 4 A 1 4 1 3 2 2 - - - - - 0 3-hexanone liquid 1 B 1 1 1 0 0 0 0 0 3 3 - - - - - 0 4-heptanone liquid 1 A 1 1 1 A 0 0 0 B 3 3 - - - - - + acetaldehyde liquid 1 1 1 1 B 0 0 0 A 0 6 6 0 0 0 - - + acetic acid anhydride liquid 6 3 3 3 3 2 A B 1 B 2 6 6 + + + + + + acetic acid, 10 % liquid 6 6 6 6 6 6 6 6 6 6 6 6 6 0 + + + + + acetic acid, 50 % liquid 5 4 6 6 6 2 4 6 6 6 6 - - - - 0 + acetic acid, conc. -

United States Pmao" ICC Patented June 30, '1959 1 2 Adsorption on Manganese Dioxide, but It May on the 2,892,677 Other Hand He Left in the Solution
r. 2,892,677 United States Pmao" ICC Patented June 30, '1959 1 2 adsorption on manganese dioxide, but it may on the 2,892,677 other hand he left in the solution. If it is removed, a certain amount of manganese nitrate is formed from the ' SEPARATION OF URANIUM FROM THORIUM manganese dioxide in the solution and this nitrate reacts AND PROTACTINIUM with the sodium diethyldithiocarbamate to give manga William Kenneth Rodgerson Musgrave, Durham, Eng ' nese diethyldithiocarbamate, which must subsequently be land, assignor, by mesne assignments, to the United separated from the uranium. ,, . .States of America as represented by the United States Whether or not the protactinium has been removed, Atomic Energy Commission the solution is brought to a pH of between 2 and 3, for No Drawing. Application November 27, 1946 10 example by the addition of ammonia. The sodium di Serial No. 712,722 ethyldithiocarbamate is then dissolved in a solvent in which the subsequently formed uranyl diethyldithiocar '8 Claims. (Cl. 23—14.5) bamate is also soluble. Such a solvent is, for example, amyl acetate or methyl isobutyl ketone, the former being This invention relates to the separation of uranium 15 preferable for the reasons which will be indicated below. from thorium and protactinium. A mixture of these The sodium diethyldithiocarbamate is dissolved in amyl elements is obtained, for example, as the result of irradia acetate to form a solution containing 0.25% of the tion by neutrons of so-called thorium carbonate, which former, and this solution is then shaken up with the is a mixture of thorium oxide and thorium carbonate. -

Solvent Extraction of Uranium with Acetylacetone and Tri-N-Butyl
DAEHAN HWAHAK HWOEJEE (Journal of the Korean Chemical Society) Vol. 24, No. 3, 1980 Printed in the Republic of Korea 아세 틸아세톤과 트리부틸인산의 도데칸용액 에 의한 우라늄의 용매추출 裴奎善-鄰奇碩 韓國核燃料開發公團 (1980. 2. 27 接受 ) Solvent Extraction of Uranium with Acetylacetone and Tri-n-Butyl Phosphate in “-Dodecane Kyu Sun Bai and Key Suck Jung Korea Nuclear Fuel Development Institute, Daeduck 300-32, Korea (Received Feb. 27, 1980) 요 악 . 도데칸에 녹인 아세틸아세톤과 트리부틸인산으로 묽은 질산우라닐수용액에서 우라늄 (VI) 을 추출했다 . pHl 이상에서 이 혼합추출제의 상승적 효과가 관측되었다 . 추출되는 화학종은 1:2:1 및 1:2:2 우라닐 -아세털 아세톤 -트리부털 인산착물이다 . 이들 반응의 추출정수들을 측정하였다 . ABSTRACT. Uranium (VI) was extracted from dilute aqueous solutions of uranyl nitrate with acetylacetone and tri-n-butyl phosphate in n-dodecane. Synergistic effect was observed with the mixed reagents above pH 1, The species extracted are the 1:2:1 and the 1:2:2 uranyl-AA-TBP complexes. The extraction constants for these reactions have been determined. by crystallization of the salt as UO2(NO3)2 INTRODUCTION •6H2O. HAA and TBP were dissolved in do Acetylacetone (HAA) is a simple -diketone. decane and uranyl nitrate in water. Equal A weak acid with pKa=8. 2% HAA has long volumes of organic and aqueous phases were been used as a chelating agent for many equilibrated in 100 mZ separatory funnels by metals.2 It is quite soluble in water and its vigorous shaking for several minutes. Equili brium temperature was 25 °C or ambient. pH distribution coefficient is E=5. 95 for benzene/ water system.3 Because of its solubility in measurements were made by using a Corning water, HAA has not been so widely used as, Model 130 pH meter. -

TRI.N.BUTYL PHOSPHATE
This report contains the collective views of an in- ternational group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the Interna- tional Labour Organisation, or the World Health Organization. Environmental Health Cnteria ll2 TRI.n.BUTYL PHOSPHATE Published under the joint sponsorshipof the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization First draft prepared by Dr A. Nakamura, National Institute for Hygienic Sciences,Japan World Health Organization Geneva,1991 The International Progranne on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objec- tive of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environ- ment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination of laboratory testing and epidemiological studies, and promotion of research on the mechanisms of the biological action of chemicals. WHO Library Cataloguing in Publication Data Tri-n-butyl phosphate. (Environmental health criteria ; l12) l. Phosphoric acid esters - adverse effects 2. Phosphoric acid esters - toxicity I. Series rsBN92 4 r57rr2 8 (NLM Classification: QV 627) rssN0250-863X @World Health Organization l99l Publications of the \ilorld Health Organization enjoy copyright pro- tection in accordance with the provisions of Protocol 2 of the Univer- sal Copyright Convention. -

Material Safety Data Sheet
SAFETY DATA SHEET URANYL NITRATE SOLUTION SECTION 1: CHEMICAL PRODUCT & COMPANY IDENTIFICATION New Brunswick Laboratory U.S. Department of Energy 9800 South Cass Avenue Argonne, IL 60439 1-630-252-CRMS (2767) Emergency Phone Numbers: 1-630-252-6130 or 1-630-252-5731 Chemical Name: Uranyl Nitrate Solution, UO2(NO3)2 Other Identifiers: Certified Reference Material (CRM) standard or Safeguards Measurement Evaluation (SME) sample Use and Restrictions: This material is prepared for use as a standard or in inter-laboratory comparison programs at analytical laboratories, which routinely handle uranium and/or plutonium. NBL expects that recipients of their material are in compliance with 29 CFR 1910.1200(h) which requires employers to provide employees with effective information and training on hazardous chemicals in their work area. SECTION 2: HAZARDS IDENTIFICATION OSHA HAZARDS: Skin Corrosion/Irritation Category 1B. Serious Eye Damage/Irritation Category 1 TARGET ORGANS: Skin, Eyes, Kidneys. GHS Label elements, including precautionary statements Signal Word: DANGER Pictogram: Page 1 of 12 Hazard Statement(s) H314 Causes severe skin burns and eye damage H373 May cause damage to organs through prolonged or repeated exposure. H411 Toxic to aquatic life with long lasting effects. Precautionary statement(s) P260 Do not breathe dust/ fume/ gas/ mist/ vapors/ spray. P262 Do not get in eyes, on skin, or on clothing. P264 Wash skin thoroughly after handling. P273 Avoid release to the environment. P280 Wear protective gloves/protective clothing/eye protection/face protection P310 Immediately call a POISON CENTER or doctor/ physician if swallowed or inhaled. Other Hazard(s): Radioactive NFPA RATINGS (SCALE 0-4): Health=3 Fire=0 Reactivity=0 Special Hazard= OX SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTS Chemical Name: Uranyl Nitrate Solution Common Names/Synonyms: CRM U045; CRM 135; CRM 145; CRM 145-B; CRM U930-D; Safeguards Measurement Evaluation (SME) Low Enrichment, Normal Enrichment, or High Enrichment Solutions; Uranyl Nitrate in Nitric Acid Solution. -

Interagency Committee on Chemical Management
DECEMBER 14, 2018 INTERAGENCY COMMITTEE ON CHEMICAL MANAGEMENT EXECUTIVE ORDER NO. 13-17 REPORT TO THE GOVERNOR WALKE, PETER Table of Contents Executive Summary ...................................................................................................................... 2 I. Introduction .......................................................................................................................... 3 II. Recommended Statutory Amendments or Regulatory Changes to Existing Recordkeeping and Reporting Requirements that are Required to Facilitate Assessment of Risks to Human Health and the Environment Posed by Chemical Use in the State ............................................................................................................................ 5 III. Summary of Chemical Use in the State Based on Reported Chemical Inventories....... 8 IV. Summary of Identified Risks to Human Health and the Environment from Reported Chemical Inventories ........................................................................................................... 9 V. Summary of any change under Federal Statute or Rule affecting the Regulation of Chemicals in the State ....................................................................................................... 12 VI. Recommended Legislative or Regulatory Action to Reduce Risks to Human Health and the Environment from Regulated and Unregulated Chemicals of Emerging Concern .............................................................................................................................. -

Uranyl Nitrate, 1%
Uranyl Nitrate, 1% 1. Identification Product Name: Uranyl Nitrate, 1% Item #: DI0103014, DI0103029, SKC1048-250, SKC1048-500 Web SDS: S231 Synonyms: N/A Recommended Use: Special Stains Restrictions on Use: N/A Manufacturer: In Case of Emergency: BBC Biochemical Chemtrec US 1-800-424-9300 409 Eleanor Lane, Chemtrec International 703-527-3887 Mount Vernon, WA 98273 1-800-635-4477 2. Hazards Identification OSHA Hazard Classification(s): Acute Toxicity - Oral - Category 4 Acute Toxicity - Inhalation - Category 4 Signal Word: Warning Hazard Statement(s): Harmful if swallowed. Harmful if inhaled. Pictogram(s): Precautionary Statement(s): Prevention: Wash body thoroughly after handling. Do not eat, drink or smoke when using this product. Avoid breathing dust, vapors. Use only outdoors or in a well-ventilated area. Response: If swallowed: Call a doctor if you feel unwell. Rinse mouth. If inhaled: Remove person to fresh air and keep comfortable for breathing. Call a doctor if you feel unwell. Storage: N/A Disposal: Dispose of contents/container in accordance with local regulations. Descriptions of Hazards not otherwise classified: N/A Percent of mixture with unknown acute toxicity: N/A 3. Composition and Information on Ingredients Chemical Name Common Name CAS # Concentration % Uranyl Nitrate 13520-83-7 1 Water 7732-18-5 99 4. First Aid Measures Eye Contact: If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. Skin Contact: If on skin (or hair): Take off immediately all contaminated clothing and wash before reuse. -

Physcio-Chemical Studies on the Systems Uranyl Nitrate Organic Solvent
Durham E-Theses Physcio-chemical studies on the systems uranyl nitrate organic solvent - water Mathieson A. R., How to cite: Mathieson A. R., (1951) Physcio-chemical studies on the systems uranyl nitrate organic solvent - water, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/9766/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk PSYSIC0-CH3MICAL STUDIES OH THE SYSTEMS DEAUTL 1TITRATE - ORGANIC SOLVENT - WATER A.R. Mathieson, B.Sc.(Dunelm), A.R.I.C. Thesis presented for the degree of M. Sc. in Pure Science of the University of Durham, August 1951 The experimental work described in this thesis was performed at the Atomic Energy Research Establishment, Harwell, during the period November 1947 - December 1949* The results have already been published elsewhere: Mathieson "Stability of Complexes of Uranyl Nitrate with Ketones and Ethers", J. -

Tributyl Phosphate Cas N°: 126-73-8
OECD SIDS TRIBUTYL PHOSPHATE FOREWORD INTRODUCTION TRIBUTYL PHOSPHATE CAS N°: 126-73-8 UNEP PUBLICATIONS 1 OECD SIDS TRIBUTYL PHOSPHATE SIDS Initial Assessment Report for 12th SIAM (Paris, France June 2001) Chemical Name : Tributyl Phosphate CAS No: 126-73-8 Sponsor Country: U.S.A National SIDS Contact Point in Sponsor Country: US EPA Dr. Oscar Hernandez (7403M) ICC Building 1200 Pennsylvania Avenue, N.W. Washington, DC 20460 U.S.A. e-mail: [email protected] phone: 202-564-7641 HISTORY: First time considered for a SIAM. No new testing was performed. All available data was provided from company files and public databases. COMMENTS: An IPCS document is available for this chemical. IPCS Environmental Health Criteria Document No. 112, WHO, 1991. Deadline for circulation: Date of Circulation: April 2001 (updated March 2002) 2 UNEP PUBLICATIONS OECD SIDS TRIBUTYL PHOSPHATE SIDS INITIAL ASSESSMENT PROFILE CAS No. 126-73-8 Chemical Name Tributyl phosphate Structural Formula (C4H9O)3PO RECOMMENDATIONS The chemical is a candidate for further work under conditions specified below. SUMMARY CONCLUSIONS OF THE SIAR Human Health The toxicology database for tributyl phosphate (TBP) is large and well documented. There are adequate data with which to evaluate the potential hazard to human health of this compound. Acute oral toxicity values in rodents range from 1390 to 3350 mg/kg-bw in rats and from 400 to 1240 mg/kg-bw in mice. A rat six-hour LC50 of > 4.2 mg/L (highest dose tested) was reported. Dermal studies exist in rabbits (LD50s of >3100 mg/kg-bw and > 10,000 mg/kg-bw) and in guinea pigs (LD50 of 9700 – 19,400 mg/kg-bw). -

Hand Protection
HAND PROTECTION CHEMICAL This table provides only general information. Be careful ! Glove resist- ance is influenced by other factors such as temperature, chemical RESISTANCE TABLE product concentration, thickness, immersion time, and others. For specific use conditions, we recommend testing the glove prior to use. n°CAS Natural latex Neoprene Nitrile vinyl PVC n°CAS Natural latex Neoprene Nitrile vinyl PVC 20% nitric acid 7697-37-2 ** ** * * Isobutyl alcohol 78-83-1 *** *** *** *** 30% and 5% hydrochloric acid 7647-01-0 *** *** *** ** Isobutyl ketone / *** *** 30% formaldehyde 50-00-0 *** *** *** *** Kerosene / * *** * 30% hydrofluoric acid 7664-39-3 ** *** *** ** Lard oil / *** *** * 50% acetic anhydride / *** *** *** *** Linseed oil / *** *** * 85% lactic acid / * *** *** *** Lubricating oils / * *** * 85% triethanolamine 102-71-6 *** *** *** *** Magnesia 1309-48-4 *** *** *** *** 90% formic acid 64-18-6 *** * * Methyl acetate 79-20-9 * *** * * Acetaldehyde 75-07-0 *** *** * Methyl alcohol (or methanol) 67-56-1 *** *** *** *** Acetone 67-64-1 *** ** Methyl ethyl ketone / *** ** Alcoholic beverages / *** *** *** *** Methyl isobutyl ketone / ** * Ammonium acetate 631-61-8 *** *** *** *** Methyl salicylate 119-36-8 *** *** *** *** Ammonium carbonate 10361-29-2 *** *** *** *** Methylamine 74-89-5 *** ** *** *** Ammonium chloride 12125-02-9 *** *** *** *** Methylaniline 100-61-8 * * *** *** Amyl acetate / *** Methylcyclopentane 96-37-7 * *** * Amyl alcohol 71-41-0 *** *** *** *** Methylene chloride 75-09-2 ** Aniline 62-53-3 ** ** * Methylformiate