Ammonium Diuranate' Prepared from Uranium Hexa¯Uoride J.A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Extraction Processing of Concentrated Solutions of Uranyl Nitrate with High Impurities Content V.M
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Electronic archive of Tomsk Polytechnic University Сhemistry UDC 546.791.02.238:66.061.51 EXTRACTION PROCESSING OF CONCENTRATED SOLUTIONS OF URANYL NITRATE WITH HIGH IMPURITIES CONTENT V.M. Korotkevich, V.V.Lazarchuck, T.G. Shikerun, V.I. Shamin, N.A. Mikhailova, F.A. Dorda Federal Unitary Enterprise Siberian Group of Chemical Enterprises, Seversk Еmail: [email protected] Process flowsheet of recycling uranium concentrated solutions with its purification from insoluble impurities of iron, silicon, molybde num, calcium oxides and hydroxides and soluble impurities with application of centrifugal extractors cascade has been developed and suggested for commercial introduction. The process was carried out at extractant saturation (30 % tributyl phosphate in hydrocarbon diluent) in extraction assembly lower than a limiting level (85...95 g/l) and in wash assembly – at limiting saturation (up to 120 g/l). As a result the waste uranium content in watertail solutions 0,01...0,04 g/l and minimal content of impurities in reextractors is provided. Perspective program on development of nuclear technology are also: reliability of hermetization, possi power engineering proposes to involve wide range of raw bility of remote service, resistance to corrosive attack of materials from uranium ores to secondary uranium ma structural materials, duration of overhaul period etc. terials into processing at atomic enterprises. In this case In Russia the centrifugal extractor of ECТseries the problem of reprocessing concentrated uranium so with continuous extraction of solid phase [3], which may lutions with high impurity content occurs. -

Key Official US and IAEA Statements About Iran's Nuclear Programs
Key Official US and IAEA Statements About Iran’s Nuclear Programs Anthony H. Cordesman There is a great deal of speculation about Iran’s nuclear programs that do not list sources or reflect the views of the US intelligence community. It is worth examining what top US intelligence official have said during the last few years, and the details of the IAEA report published in November 2011 – one that clearly reflected official inputs from the US and a number of European intelligence services. U.S. Official Statements on the Iranian Nuclear and Missile Threat The annual unclassified reports to Congress by the Office of the Director of National Intelligence are a key source of US official view. Although the 2010 report by James R. Clapper has already been partly overtaken by the pace of Iran’s rapidly developing program, it still represents the most detailed unclassified estimate of Iran’s capabilities by a senior US official:1 Nuclear We continue to assess Iran is keeping open the option to develop nuclear weapons though we do not know whether Tehran eventually will decide to produce nuclear weapons. Iran continues to develop a range of capabilities that could be applied to producing nuclear weapons, if a decision is made to do so. During the reporting period, Iran continued to expand its nuclear infrastructure and continued uranium enrichment and activities related to its heavy water research reactor, despite multiple United Nations Security Council Resolutions since late 2006 calling for the suspension of those activities. Although Iran made progress in expanding its nuclear infrastructure during 2001, some obstacles slowed progress during this period. -

Depleted Uranium Hexafluoride: Waste Or Resource?
UCRGJC-120397 PREPRINT Depleted Uranium Hexafluoride: Waste or Resource? N. Schwertz J. Zoller R Rosen S. Patton C. Bradley A. Murray This paper was prepared for submittal to the Global ‘95 International Conference on Evaluation of Emerging Nuclear Fuel Cycle Systems Versailles, France September 11-14,1995 July 1995 This isa preprint of apaper intended for publication in a jaurnal orproceedings. Since changes may be made before publication, this preprint is made available with the understanding that it will not be cited or reproduced without the permiasion of the anthor. DISCLAIMER This document was prepared as an account of work sponsored by an agency of the United Stat= Government. Neither theunited States Governmentmor theuniversity of California nor any oftheir employees, makes any warranty, express or implied, or assumesanylegalliabilityorrespomibility forthe accuracy,completeness,orusefuin~ of any information, apparatus, pduct, or process disdosed, or represents that its use wouldnotinfringe privatelyowned rights. Referencehemin to anyspe&c commercial prodocis, proms, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constituteor imply its endorsement, reconunendation, or favoring by the United States Government or the University of California. The views and opinions of authors expressed herein do not necessady state or reflect those of the United States Government or the University of California, and shall not be used for adveltising or product endorsement purposes. DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document . DEPLETED URANIUM HEXAFLUORIDE: WASTE OR RESOURCE? N. Schwertz, J. Zoller, R. Rosen, S. Patton LAWRENCE LIVERMORE NATIONAL LABORATORY P. -
Nitrate (UO2 (NO)) 4
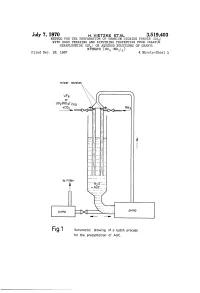
July 7, 1970 H. VETZKE ETA 3,519,403 METHOD FOR THE PREPARATION OF URANIUM DIOXIDE POWDER (UO) WITH GOOD PRESSING AND SINTERING PROPERTIES FROM URANIUM HEXAFLUORIDE (UF) OR AQUEOUS SOLUTIONS OF URANYL Fied Dec. 18, 1967 NiTRATE (UO2 (NO)) 4. Sheets-Sheet l mixer nozzles --race to fitter Fig.1 Schematic drawing of a batch process for the precipitation of AUC. July 7, 1970 H. VETZKE EA 3,519,403 METHOD FOR THE PREPARATION OF URANIUM DIOXIDE POWDER (UO) WITH GOOD PRESSING AND SINTERING PROPERTIES FROM RANIUM HEXAFLUORIDE (UF) OR AQUEOUS SOLUTIONS OF URANYL Filed Dec. 8, 1967 NiTRATE (UO2 (NO)) 4. Sheets-Sheet 3. mixer nozzles UF6 Of U02 (NO3)2ad tC92--X Ex-Ns / to filter cooling precipitation Vessel vessel Fig. 2 Schematic drawing of a continuous process for the precipitation of AUC July 7, 1970 H, VETZKE ETAL 3,519,403 METHOD FOR THE PREPARATION OF URANIUM DIOXIDE POWDER (UO) WITH GOOD PRESSING AND SINTERING PROPERTIES FROM URANIUM HEXAFLUORIDE (UF) OR AQUEOUS SOLUTIONS OF URANYL Filed Dec. 18, 1967 NiTRATE (UO (NO)2) 4. Sheets-Sheet 3 Off gas Off gas 487\m Nx UO2 powder XXX.& X.X S& steam / H2 Fig. 3 Ot Schematic drawing of a fluidized bed furnace for the reduction of AUC to U02, July 7, 1970 H. VEZKE ETAL 3,519,403 METHOD FOR THE PREPARATION OF URANIUM DIOXIDE POWDER (UO) WITH GOOD PRESSING AND SINTERING PROPERTIES FROM URANIUM HEXAFLUORIDE (UF) OR AQUEOUS SOLUTIONS OF URANYL NITRATE (UO2 (NO)a) Filed Dec. 18, 1967 4. Sheets-Sheet 1 -- Offgas Step 1: Decomposition, reduction and pyrohydrolysis i Step 2: Pyrohydrolysis Step 3: Controlled oxidation an o- are as an un- Fig. -

Uranium Aerosols at a Nuclear Fuel Fabrication Plant: Characterization Using Scanning Electron Microscopy and Energy Dispersive X-Ray Spectroscopy
Spectrochimica Acta Part B 131 (2017) 130–137 Contents lists available at ScienceDirect Spectrochimica Acta Part B journal homepage: www.elsevier.com/locate/sab Uranium aerosols at a nuclear fuel fabrication plant: Characterization using scanning electron microscopy and energy dispersive X-ray spectroscopy E. Hansson a,b,⁎, H.B.L. Pettersson a, C. Fortin c, M. Eriksson a,d a Department of Medical and Health Sciences, Linköping University, 58185 Linköping, Sweden b Westinghouse Electric Sweden AB, Bränslegatan 1, 72136 Västerås, Sweden c Carl Zeiss SAS, 100 route de Versailles, 78160 Marly-le-Roi, France d Swedish Radiation Safety Authority, 17116 Stockholm, Sweden article info abstract Article history: Detailed aerosol knowledge is essential in numerous applications, including risk assessment in nuclear industry. Received 31 March 2016 Cascade impactor sampling of uranium aerosols in the breathing zone of nuclear operators was carried out at a Received in revised form 28 February 2017 nuclear fuel fabrication plant. Collected aerosols were evaluated using scanning electron microscopy and energy Accepted 3 March 2017 dispersive X-ray spectroscopy. Imaging revealed remarkable variations in aerosol morphology at the different Available online 6 March 2017 workshops, and a presence of very large particles (up to≅100 × 50 μm2) in the operator breathing zone. Charac- teristic X-ray analysis showed varying uranium weight percentages of aerosols and, frequently, traces of nitrogen, Keywords: fl Uranium uorine and iron. The analysis method, in combination with cascade impactor sampling, can be a powerful tool Aerosol for characterization of aerosols. The uranium aerosol source term for risk assessment in nuclear fuel fabrication Impactor appears to be highly complex. -

United States Pmao" ICC Patented June 30, '1959 1 2 Adsorption on Manganese Dioxide, but It May on the 2,892,677 Other Hand He Left in the Solution
r. 2,892,677 United States Pmao" ICC Patented June 30, '1959 1 2 adsorption on manganese dioxide, but it may on the 2,892,677 other hand he left in the solution. If it is removed, a certain amount of manganese nitrate is formed from the ' SEPARATION OF URANIUM FROM THORIUM manganese dioxide in the solution and this nitrate reacts AND PROTACTINIUM with the sodium diethyldithiocarbamate to give manga William Kenneth Rodgerson Musgrave, Durham, Eng ' nese diethyldithiocarbamate, which must subsequently be land, assignor, by mesne assignments, to the United separated from the uranium. ,, . .States of America as represented by the United States Whether or not the protactinium has been removed, Atomic Energy Commission the solution is brought to a pH of between 2 and 3, for No Drawing. Application November 27, 1946 10 example by the addition of ammonia. The sodium di Serial No. 712,722 ethyldithiocarbamate is then dissolved in a solvent in which the subsequently formed uranyl diethyldithiocar '8 Claims. (Cl. 23—14.5) bamate is also soluble. Such a solvent is, for example, amyl acetate or methyl isobutyl ketone, the former being This invention relates to the separation of uranium 15 preferable for the reasons which will be indicated below. from thorium and protactinium. A mixture of these The sodium diethyldithiocarbamate is dissolved in amyl elements is obtained, for example, as the result of irradia acetate to form a solution containing 0.25% of the tion by neutrons of so-called thorium carbonate, which former, and this solution is then shaken up with the is a mixture of thorium oxide and thorium carbonate. -

Solvent Extraction of Uranium with Acetylacetone and Tri-N-Butyl
DAEHAN HWAHAK HWOEJEE (Journal of the Korean Chemical Society) Vol. 24, No. 3, 1980 Printed in the Republic of Korea 아세 틸아세톤과 트리부틸인산의 도데칸용액 에 의한 우라늄의 용매추출 裴奎善-鄰奇碩 韓國核燃料開發公團 (1980. 2. 27 接受 ) Solvent Extraction of Uranium with Acetylacetone and Tri-n-Butyl Phosphate in “-Dodecane Kyu Sun Bai and Key Suck Jung Korea Nuclear Fuel Development Institute, Daeduck 300-32, Korea (Received Feb. 27, 1980) 요 악 . 도데칸에 녹인 아세틸아세톤과 트리부틸인산으로 묽은 질산우라닐수용액에서 우라늄 (VI) 을 추출했다 . pHl 이상에서 이 혼합추출제의 상승적 효과가 관측되었다 . 추출되는 화학종은 1:2:1 및 1:2:2 우라닐 -아세털 아세톤 -트리부털 인산착물이다 . 이들 반응의 추출정수들을 측정하였다 . ABSTRACT. Uranium (VI) was extracted from dilute aqueous solutions of uranyl nitrate with acetylacetone and tri-n-butyl phosphate in n-dodecane. Synergistic effect was observed with the mixed reagents above pH 1, The species extracted are the 1:2:1 and the 1:2:2 uranyl-AA-TBP complexes. The extraction constants for these reactions have been determined. by crystallization of the salt as UO2(NO3)2 INTRODUCTION •6H2O. HAA and TBP were dissolved in do Acetylacetone (HAA) is a simple -diketone. decane and uranyl nitrate in water. Equal A weak acid with pKa=8. 2% HAA has long volumes of organic and aqueous phases were been used as a chelating agent for many equilibrated in 100 mZ separatory funnels by metals.2 It is quite soluble in water and its vigorous shaking for several minutes. Equili brium temperature was 25 °C or ambient. pH distribution coefficient is E=5. 95 for benzene/ water system.3 Because of its solubility in measurements were made by using a Corning water, HAA has not been so widely used as, Model 130 pH meter. -

Comparative Study on Precipitation Methods of Yellow
Precipitation and purification of uranium from rock phosphate Elshafeea H. Y. Abow Slama1, Etemad Ebraheem2 and Adam K. Sam1 1Sudan Atomic Energy Commission P.O. Box 3001, Khartoum-Sudan 2 Sinner University ABSTRACT This study was carried-out to leach uranium from rock phosphate using sulphuric acid in the presence of potassium chlorate as an oxidant and to investigate the relative purity of different forms of yellow cakes produced with ammonia {( NH 4 )2 U2O7 }, magnesia (UO3.xH 2O ) and sodium hydroxide Na2U 2O7 as precipitants, as well as purification of the products with TBP extraction and matching its impurity levels with specifications of the commercial products. Alpha-particle spectrometry was used for determination of activity concentration of uranium isotopes (234U and 238U) in rock phosphate, resulting green phosphoric acid solution, and in different forms of the yellow cake from which the equivalent mass concentration of uranium was deduced. Likewise, atomic absorption spectroscopy (AAS) was used for determination of impurities (Pb, Ni, Cd, Fe, Zn, Mn, and Cu). On the average, the equivalent mass concentration of uranium was 119.38±79.66 ppm (rock phosphate) and 57.85±20.46 ppm (green solution) with corresponding low percent of dissolution (48%) which is considered low. The isotopic ratio (234U: 238U) in all stages of hydrometallurgical process was not much differ from unity indicating lack of fractionation. Upon comparing the levels of impurities in different form of crude yellow cakes, it was found that the lowest levels were measured in UO3.xH2O. This implies that saturated magnesia is least aggressive relative to other precipitants and gives relatively pure crude cake. -

Nuclear Iran a Glossary of Terms
Nuclear Iran A Glossary of Terms Simon Henderson and Olli Heinonen Policy Focus 121 | May 2013 Update HARVARD Kennedy School A COPUBLICATION WITH BELFER CENTER for Science and International Affairs Map: Nuclear installations in Iran. TURKMENISTAN TABRIZ Bonab Lashkar Abad TEHRAN MASHAD Karaj Marivan Parchin Fordow Arak QOM IRAQ Natanz AFGHANISTAN Isfahan Ardakan Saghand Darkhovin Yazd IRAN KUWAIT SHIRAZ Bushehr PAKISTAN Gchine BANDAR ABBAS BAHRAIN SAUDI ARABIA QATAR © 2012 The Washington Institute for Near East Policy UAE OMAN Map: Nuclear installations in Iran. Nuclear Iran A Glossary of Terms Simon Henderson and Olli Heinonen Policy Focus 121 | May 2013 Update HARVARD Kennedy School A COPUBLICATION WITH BELFER CENTER for Science and International Affairs n n n The authors extend special thanks to Mary Kalbach Horan and her editorial team at The Washington Institute. n n n All rights reserved. Printed in the United States of America. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording, or any information storage and retrieval system, without permission in writing from the publisher. © 2012, 2013 by The Washington Institute for Near East Policy and the Harvard Kennedy School’s Belfer Center for Science and International Affairs Copublished in 2012 and 2013 in the United States of America by The Washington Institute for Near East Policy, 1828 L Street NW, Suite 1050, Washington, DC 20036; and the Harvard Kennedy School’s Belfer Center for Science and International Affairs, 79 JFK St., Cambridge, MA 02138. Cover photo: Iran’s president Mahmoud Ahmadinejad visits the Natanz nuclear enrichment facility. -

Sorption of Np and Tc in Underground Waters by Uranium Oxides
Sorption of Np and Tc in Underground Waters by Uranium Oxides T.V.Kazakovskaya,a V.I. Shapovalova, N.V. Kushnira , E.V. Zakharovab, S.N.Kalmykovb O.N.Batukb, M.J.Hairec a –Russian Federal Nuclear center –All-Russia Scientific Institute of Experimental Physics; 607190, Sarov, Russia, E-mail: [email protected]; b – Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, Moscow, Russia, c – Oak Ridge National Laboratory, Oak Ridge, Tennessee. Abstract –The production of nuclear fuels results in the accumulation of large quantities of depleted uranium (DU) in the form of uranium hexafluoride (UF6), which is converted to uranium oxides. Depleted uranium dioxide (DUO2) can be used as a component of radiation shielding and as an absorbent for migrating radionuclides (especially 237Np and 99Tc). These may emerge from casks containing spent nuclear fuel (SNF) that are stored for hundreds of thousands of years in high-level wastes (HLW) and SNF repositories (e.g. Yucca Mountain Project). In this case DU oxides serve as an additional engi- neered chemical barrier. This work is a part of the joint Russian –American Program on Beneficial Use of Depleted Uranium. This paper describes the UO2 transformations that take place in contact with various aqueous media (deionized water (DW), J-13 solution that simulates Yucca Mountain ground water, etc.) and sorption of long-lived radionuclides (237Np and 99Тс) from these media by depleted uranium dioxide. Samples of depleted uranium dioxide used in this work originated from the treatment of UF6 in a reducing media to form UO2 (DUO2-1 at 600°C, DUO2-2 at 700°C, and DUO2-3 at 800°C). -

Material Safety Data Sheet
SAFETY DATA SHEET URANYL NITRATE SOLUTION SECTION 1: CHEMICAL PRODUCT & COMPANY IDENTIFICATION New Brunswick Laboratory U.S. Department of Energy 9800 South Cass Avenue Argonne, IL 60439 1-630-252-CRMS (2767) Emergency Phone Numbers: 1-630-252-6130 or 1-630-252-5731 Chemical Name: Uranyl Nitrate Solution, UO2(NO3)2 Other Identifiers: Certified Reference Material (CRM) standard or Safeguards Measurement Evaluation (SME) sample Use and Restrictions: This material is prepared for use as a standard or in inter-laboratory comparison programs at analytical laboratories, which routinely handle uranium and/or plutonium. NBL expects that recipients of their material are in compliance with 29 CFR 1910.1200(h) which requires employers to provide employees with effective information and training on hazardous chemicals in their work area. SECTION 2: HAZARDS IDENTIFICATION OSHA HAZARDS: Skin Corrosion/Irritation Category 1B. Serious Eye Damage/Irritation Category 1 TARGET ORGANS: Skin, Eyes, Kidneys. GHS Label elements, including precautionary statements Signal Word: DANGER Pictogram: Page 1 of 12 Hazard Statement(s) H314 Causes severe skin burns and eye damage H373 May cause damage to organs through prolonged or repeated exposure. H411 Toxic to aquatic life with long lasting effects. Precautionary statement(s) P260 Do not breathe dust/ fume/ gas/ mist/ vapors/ spray. P262 Do not get in eyes, on skin, or on clothing. P264 Wash skin thoroughly after handling. P273 Avoid release to the environment. P280 Wear protective gloves/protective clothing/eye protection/face protection P310 Immediately call a POISON CENTER or doctor/ physician if swallowed or inhaled. Other Hazard(s): Radioactive NFPA RATINGS (SCALE 0-4): Health=3 Fire=0 Reactivity=0 Special Hazard= OX SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTS Chemical Name: Uranyl Nitrate Solution Common Names/Synonyms: CRM U045; CRM 135; CRM 145; CRM 145-B; CRM U930-D; Safeguards Measurement Evaluation (SME) Low Enrichment, Normal Enrichment, or High Enrichment Solutions; Uranyl Nitrate in Nitric Acid Solution. -

DEPLETED URANIUM HEXAFLUORIDE (Current Situation, Safe Handling and Prospects)
DEPLETED URANIUM HEXAFLUORIDE (current situation, safe handling and prospects) 2020 Authors: Alexander Nikitin, Head of Bellona Foundation Oleg Muratov, nuclear physicist, Head of Radiation Technology Dept., TVEL JSC Ksenia Vakhrusheva, Cand. Sci. Econ., Expert, BELLONA International Foundation Editor: Elena Verevkina Design: Alexandra Solokhina This Report was prepared with support and participation of Environmental Board of the Public Council of Rosatom State Atomiс Energy Corporation Publishers: Bellona Foundation Environmental Protection NGO ‘Ecopravo’ Expert and Legal Center TABLE OF CONTENTS Abbreviations ............................................................................................................................. 4 Foreword ..................................................................................................................................... 5 Introduction ................................................................................................................................ 6 Chapter 1. A few words about nuclear physics and radioactivity for non-specialists............... 8 Chapter 2. Uranium hexafluoride and its properties ..................................................................... 11 2.1. Physical properties of uranium hexafluoride .................................................................. 12 2.2. Chemical properties of uranium hexafluoride ................................................................ 13 Chapter 3. What is DUHF ..................................................................................................................