Characterization of a New Glucomannan from Eremurus Spectabilis Roots
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cibulnaté a Hlíznaté Rostliny
Cibulnaté a hlíznaté rostliny Přehled druhů 2: Asparagales Řád Asparagales rozsáhlý řád, 14 čeledí, některé obrovské semena rostlin obsahují černé barvivo melanin (některé druhy ho druhotně ztratily) Hosta PREZENTACE © JN Iridaceae (kosatcovité) Řád Asparagales Čeleď Iridaceae (kosatcovité) vytrvalé byliny s oddenky, hlízami, nebo cibulemi stonek přímý nevětvený, někdy zkrácený listy mečovité nebo čárkovité, dvouřadé se souběžnou žilnatinou květy jednotlivé nebo v chudých květenstvích (vějířek nebo srpek) – významné druhy okrasného zahradnictví subtropy až mírné pásmo 70/1750, ČR 3/12 PREZENTACE © JN Iridaceae (kosatcovité) Řád Asparagales Čeleď Iridaceae (kosatcovité) Zahradnicky významné jsou: mečíky (Gladiolus), frézie (Freesia), kosatce (Iris), šafrány (Crocus) Mezi další zahradnicky významné Iridaceae patří např. Crocosmia, Ixia, Tigridia © Saxifraga-Dirk Hilbers © Saxifraga-Inigo Sanchez Iris xiphium http://www.freenatureimages.eu/Plants/Flora%20D-I/Iris%20xiphium/slides/Iris%20xiphium%201,%20Saxifraga-Dirk%20Hilbers.jpg http://www.freenatureimages.eu/Plants/Flora%20D-I/Iris%20xiphium/slides/Iris%20xiphium%202,%20Saxifraga-Inigo%20Sanchez.jpg Iridaceae (kosatcovité) Iris (kosatec) zahrnuje i množství druhů které se neřadí mezi cibuloviny. Do cibulovin patří kosatce sekce Xiphium a Reticulata Sekce Xiphium - původní druhy pocházejí ze středomoří, hlavně Pyrenejí, zde rostou v 1500 m na mořem Cibule se 3-5 masitými šupinami, žlábkovité listy , stvol s 2-3 tuhými zelenými listeny a 2-3 květy, jsou modré se žlutým středem na vnějších okvětních lístcích, v přírodě kvetou koncem června Křížením původních druh této sekce hlavně Iris xiphium a I. tingitana vzniklo velké množství kutivarů – označované jako Dutch iris (holandské kosatce), pěstují se tržně v mnoha barvách (od bílé, žluté, modré až po fialovou) a prodávají jako řezané květiny např. -

March 2021 ---International Rock Gardener--- March 2021
International Rock Gardener ISSN 2053-7557 Number 135 The Scottish Rock Garden Club March 2021 ---International Rock Gardener--- March 2021 A month with our usual variety of plants, places and people - thanks to our contributors, we are able to bring this magazine free to all on the internet. Across the world there has been great frustration at the inability of late to travel and enjoy plants in the wild – Christopher (Chris) and Başak Gardner, planthunters, authors and organisers of Vira Natura Tours are hopeful, as are some others, of being able to resume tours soon. Meanwhile they have given us a whistle stop guide to the flowers of the Silk Road (ISBN-10: 1472969103 ISBN-13: 978-1472969101 – the subject of their substantial – and very beautiful – book, Flora of the Silk Road. The cover of their latest book, the Flora of the Mediterranean (ISBN-10: 1472970268 ISBN-13: 978-1472970268) is also shown below. Also this month: Wim Boens from Flanders appeals for assistance in the clarification of a long-running confusion over the correct identity of a fine old Colchicum cultivar. Can you help? From Chile, John and Anita Watson bring a change of rank for the infraspecific taxon of Mutisia subulata Ruiz & Pav. and also clarification of the species' differing morphology and its vertical distribution. Cover image: Omphalodes luciliae photo Chris Gardner. Vira Natura organises Botanical Holidays and Tours. www.srgc.net Charity registered in Scotland SC000942 ISSN 2053-7557 ---International Rock Gardener--- --- Plant Naming --- A change of rank for the infraspecific taxon of Mutisia subulata Ruiz & Pav. -

The Genus Cardiocrinum: Its Identification and Cultivation
The Genus Cardiocrinum: its identification and cultivation Philip Bolt The right of Philip Bolt to be identified as the author of this work have been asserted by him in accordance with the Copyright, Designs and Patent Act, 1988. First edition: November, 2016 Second edition: January 2018 The revisions take into account the results published in the paper by Li-Qin Yang, Hao-Yu Hu, Chuan Xie, Shan-Pan Lai, Mei Yang, Xing-Jin He and Song-Dong Zhou in the Annals of Botany , [LHCSMXS], 1 and C. cathayanum (hort.) is designated as C. giganteum yunnanense. Third edition: September, 2018 trnL barcode comparisons have been removed and an analysis made of variations in the matK sequences for C. giganteum yunnanense Fourth edition: November, 2018 A chapter has been added on the problems and pitfalls associated with the purchase of Cardiocrinum bulbs and seeds. The identification key has also been been modified to comply with my current knowledge of C. cathayanum. Fifth edition August, 2020 Information has been added on C. giganteum yunnanense f. rosea and C. cordatum cordatum ‘Red flowered form’. © Philip Bolt, 2016 - 2020 1 Li-Qin Yang, Hao-Yu Hu, Chuan Xie, Shan-Pan Lai, Mei Yang, Xing-Jin He and Song-Dong Zhou, Molecular phylogeny, biogeography and ecological niche modelling of Cardiocrinum (Liliaceae): insights into the evolutionary history of endemic genera distributed across the Sino-Japanese floristic region, Annals of Botany 119: 59–72, 2017 i ACKNOWLEDGEMENTS I'm grateful to my wife, Moira Bolt, for her help in maintaining the collection, to Julia Llewellyn-Hughes, DNA Sequencing Facility Manager, and her staff at The Natural History Museum, London for the sequencing of the DNA and to Sven Landrein, (Horticultural Botanist), & László Csiba, (DNA Bank Manager), formerly of the Royal Botanic Garden, Kew for their help and information about C. -
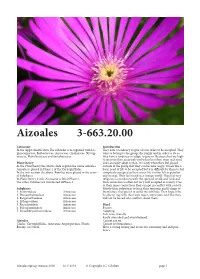
Aizoales 3-663.20.00
Aizoales 3-663.20.00 Taxonomy Introduction In the Apg2 classifcation Te suborder is recognised with Lo- Tey have a tendency to give a lot in order to be accepted. Tey phiocarpaceae, Barbeuiaceae, Aizoaceae, Gisekiaceae, Nyctag- want to belong to the group, the family and in order to do so inaceae, Phytolaccaceae and Sarcobataceae. they have a tendency to adapt, to give in. Because they are high- ly sensitive they accurately feel what the others want and need Plant theory and can easily adapt to that. It is only when they feel placed In the Plant theory the above clade is given the name Aizoales. outside of the group that they can become angry. It feels like a Aizoales is placed in Phase 2 of the Caryophyllidae. basic need of life to be accepted but it is difcult for them to feel In the frst version the above Families were placed in the sever- completely accepted as their inner life is ofen felt as peculiar al Subphases. and strange. Tey feel weird in a strange world. Tey feel very In Plant theory 2 only Aizoaceae is lef inPhase 2. religious, a connection with the spiritual world and God and Te other Families are transferred toPhase 3. that connection is ofen not very well accepted in society. Due to their inner convictions they can get in confict with society. Subphases Mostly their solution is to keep their opinions and feelings to 1. Sesuvioideae Aizoaceae themselves; they prefer to avoid the conficts. Tey hope to be 2. Drosanthemoideae Aizoaceae be able to stay with their own inner convictions and that they 3. -

The Parasitic Fungi of Ohio Plants Dissertation
THE PARASITIC FUNGI OF OHIO PLANTS DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio S tate U n iversity By CLAYTON WAYNE ELLETT, B .S ., M.Sc. The Ohio S tate U n iversity 1955 Approved by: C o-^dviser Department of Botany and Plant Pathology AC KKOWLED GBMENT S The writer sincerely thanks Dr. C. C. Allison and Dr. W. G. Stover for their counsel and encouragement throughout his years of graduate education. The writer is also indebted to Dr. H, C. Young of the Department of Botany and Plant Pathology, Ohio Agricultural Experi ment Station, and Dr. G. T. Jones, Department of Botany, Oberlin College, for permission to study the herbarium specimens at their respective institutions. The writer thanks his wife for her help in the preparation of the manuscript and for her encouragement during the course of the investigations. i i TABLE OP CONTENTS INTRODUCTION.................................................................. 1 HISTORICAL SKETCH..................... 3 METHODS OF STUDY.......................................................... 5 LIST OF OHIO FUNGI PARASITIC ON PLANTS ......... 6 DISCUSSION AND SUMMARY ..........................................ll<0 APPENDIX . o . o . ........................................................143 KEY TO THE REPORTED GENERA OF OHIO FUNGI. IMPERFECT I . ll0 LITERATURE CITED . ...................................................................“.1 5 3 INTRODUCTION "if there be in the land famine, if there be pestilence, blasting, mildew, l o c u s t ---------------------------- I Kings 8»37 Fungi have been present upon the earth for a long time, much longer than seed plants. Seward ( 3 6 ) states: "We can safely say that bacteria and many other fungi are entitled to be included among the most an cien t members of th e p la n t kingdom.” The number of known species of fungi is reported from 3U.000 to as high as 100,000 (5 , 28). -

Latin for Gardeners: Over 3,000 Plant Names Explained and Explored
L ATIN for GARDENERS ACANTHUS bear’s breeches Lorraine Harrison is the author of several books, including Inspiring Sussex Gardeners, The Shaker Book of the Garden, How to Read Gardens, and A Potted History of Vegetables: A Kitchen Cornucopia. The University of Chicago Press, Chicago 60637 © 2012 Quid Publishing Conceived, designed and produced by Quid Publishing Level 4, Sheridan House 114 Western Road Hove BN3 1DD England Designed by Lindsey Johns All rights reserved. Published 2012. Printed in China 22 21 20 19 18 17 16 15 14 13 1 2 3 4 5 ISBN-13: 978-0-226-00919-3 (cloth) ISBN-13: 978-0-226-00922-3 (e-book) Library of Congress Cataloging-in-Publication Data Harrison, Lorraine. Latin for gardeners : over 3,000 plant names explained and explored / Lorraine Harrison. pages ; cm ISBN 978-0-226-00919-3 (cloth : alkaline paper) — ISBN (invalid) 978-0-226-00922-3 (e-book) 1. Latin language—Etymology—Names—Dictionaries. 2. Latin language—Technical Latin—Dictionaries. 3. Plants—Nomenclature—Dictionaries—Latin. 4. Plants—History. I. Title. PA2387.H37 2012 580.1’4—dc23 2012020837 ∞ This paper meets the requirements of ANSI/NISO Z39.48-1992 (Permanence of Paper). L ATIN for GARDENERS Over 3,000 Plant Names Explained and Explored LORRAINE HARRISON The University of Chicago Press Contents Preface 6 How to Use This Book 8 A Short History of Botanical Latin 9 Jasminum, Botanical Latin for Beginners 10 jasmine (p. 116) An Introduction to the A–Z Listings 13 THE A-Z LISTINGS OF LatIN PlaNT NAMES A from a- to azureus 14 B from babylonicus to byzantinus 37 C from cacaliifolius to cytisoides 45 D from dactyliferus to dyerianum 69 E from e- to eyriesii 79 F from fabaceus to futilis 85 G from gaditanus to gymnocarpus 94 H from haastii to hystrix 102 I from ibericus to ixocarpus 109 J from jacobaeus to juvenilis 115 K from kamtschaticus to kurdicus 117 L from labiatus to lysimachioides 118 Tropaeolum majus, M from macedonicus to myrtifolius 129 nasturtium (p. -

A Kertészeti Növénytan Növényismereti Anyaga, 1991) Összeállították
SZENT ISTVÁN EGYETEM KERTÉSZETTUDOMÁNYI KAR NÖVÉNYTANI TANSZÉK, SOROKSÁRI BOTANIKUS KERT A KERTÉSZETI NÖVÉNYTAN Növényismereti kompendiuma 2.32 Budapest, 2000 június 2 Ebben a jegyzékben azokat a növénytaxonokat adjuk közre rendszertani sorrendben, amelyek a KERTÉSZETI NÖVÉNYTAN 2. és 3. félévének foglalkozásán, azaz a NÖVÉNY- RENDSZERTAN; valamint a NÖVÉNYFÖLDRAJZ, TÁRSULÁSTAN ÉS ÖKOLÓGIA témájú előadások, gyakorlatok anyagának elsajátításához, továbbá az időközi beszámolók, kollokvium, stb., és a növénytani tanulmányokat lezáró növénytani szigorlathoz szükséges, annak alapját, taxonómiai kereteit képezik. A felsorolt fajok fontosságát a következő kiemelésekkel jelöljük: Vastag szedéssel jelüljök azokat a taxonokat, melyek ismerete hiányában a hallgatók nem mehetnek át a növényrendszertan és növényföldrajz vizsgákon. Normál szedéssel jelöljük a növényrendszertan és növényföldrajz tárgy törzsanyagának többi taxonját. Apró betűvel jelöltök a törzsanyaghoz nem tartozó, de a rendszer részét képező taxonokat. Az időközbeni módosításokat, a listától való esetleges eltéréseket a foglalkozásokon közöljük és a növényismereti anyag újabb átdolgozásaiban rögzítjük. Ezért az „elhanyagolható” és a „feltétlenül fontos” taxonok megjelölésétől el kellett tekintenünk. Minden fontosabb hajtásos növénycsalád neve alatt megadjuk az adott taxon legfontosabb jellemzőit. Ilyen rendszerben kérdezzük vissza a 2. félév végi gyakorlaton a növényismereti anyagot. A család feldolgozásához megadott rövidítések a következők: B: jellemző vegetatív bélyeg, habitus, -

Eremurus Robustus (Regel) Regel
Eremurus robustus (Regel) Regel Identifiants : 12913/ererob Association du Potager de mes/nos Rêves (https://lepotager-demesreves.fr) Fiche réalisée par Patrick Le Ménahèze Dernière modification le 30/09/2021 Classification phylogénétique : Clade : Angiospermes ; Clade : Monocotylédones ; Ordre : Asparagales ; Famille : Xanthorrhoeaceae ; Classification/taxinomie traditionnelle : Règne : Plantae ; Sous-règne : Tracheobionta ; Division : Magnoliophyta ; Classe : Liliopsida ; Ordre : Liliales ; Famille : Xanthorrhoeaceae ; Genre : Eremurus ; Nom(s) anglais, local(aux) et/ou international(aux) : Foxtail Lily , Shirach, Stepska lilija ; Note comestibilité : * Rapport de consommation et comestibilité/consommabilité inférée (partie(s) utilisable(s) et usage(s) alimentaire(s) correspondant(s)) : Parties comestibles : racine, feuilles{{{0(+x) (traduction automatique) | Original : Root, Leaves{{{0(+x) néant, inconnus ou indéterminés. Illustration(s) (photographie(s) et/ou dessin(s)): Autres infos : dont infos de "FOOD PLANTS INTERNATIONAL" : Distribution : C'est une plante tempérée. Il pousse dans les montagnes du Tien Shan et du Pamir. Ils se produisent dans des conditions proches du désert. Il peut pousser dans des endroits arides. Il convient aux zones de rusticité 6-9{{{0(+x) (traduction automatique). Page 1/2 Original : It is a temperate plant. It grows in the Tien Shan and Pamir mountains. They occur in near desert conditions. It can grow in arid places. It suits hardiness zones 6-9{{{0(+x). Localisation : Afghanistan, Asia, Australia, Central Asia, Kyrgyzstan, Slovenia, Tajikistan, Turkestan, Uzbekistan{{{0(+x) (traduction automatique). Original : Afghanistan, Asia, Australia, Central Asia, Kyrgyzstan, Slovenia, Tajikistan, Turkestan, Uzbekistan{{{0(+x). Notes : Il existe environ 50 espèces d'Eremurus. Aussi mis dans la famille des Asphodelaceae{{{0(+x) (traduction automatique). Original : There are about 50 Eremurus species. -
Arboretum Associates Donor Roll
Universityof Idaho PRESRT STD Arboretum Associates U.S. POSTAGE PO Box 44 3147 PAID Moscow, ID 83844-3147 UNIVERSITY OF IDAHO 1111111111111111111111111111111111111111 *PBN450* Students Find Inspiration for Desi9n in Arboretum nAugust 24, Horticulturist, Paul Warnick, met with 50 third year architecture students and three faculty members to introduce us to the Arboretum. We learned about it as a unique geographically based collection of plants from temperate landscapes from around the world, a thriving habitat for songbirds· and raptors (and mosquitoes), a quiet place set apart from our busy lives on campus, and a place used and supported by many people A series of low-lying covered spaces wind down the southwest hillside near Arboretum barn providing accessible spaces for a range of uses. in the Moscow-Pullman area Designed by Ryan Ivie. and from across the Palouse. The tour was the first step in a six-week project that would engage the students in Renew your annual contribution to the Arboretum Associates for Fiscal Year 2012 and contribute designing a pavilion for small concerts as well gatherings for events such as weddings or to your favorite project fund. Please help the Arboretum grow by renewing your annual gift for the fiscal year receptions. which began July 1, 2011. ThankYou! This is a project that has been done several times during the past. This year, Diane Armpriest, Phillip Mead and Matthew Hogan each worked with a group of 16 or 17 students. There Membership Categories were a number Active $20- $49 of short exercises Sustaining $50 - $99 City __________ State ___ Zip _____ and critiques Donor $100 - $249 during the project Fund Contribution Patron $250 - $499 and the end result Arboretum Associates $ _____ Sponsor $500- $999 was that each Centennial Endowment Fund $ _____ Life Associate $1,000 and above student made a Other $ ____ design proposal, Total Contribution $ _____ including a Please charge my MasterCard VISA physical model, Contributors receive our periodic ARnoRNOTES. -
The Way of Science
ISSN 2311-2158 The Way of Science International scientific journal № 11 (45), 2017 Founder and publisher: Publishing House «Scientific survey» The journal is founded in 2014 (March) Volgograd, 2017 ISSN 2311-2158. The Way of Science. 2017. № 11 (45). UDC 53:51+54+57+631+93:902+330+101+80+340+371+32 LBC 72 The Way of Science International scientific journal, № 11 (45), 2017 The journal is founded in 2014 (March) ISSN 2311-2158 The journal is issued 12 times a year The journal is registered by Federal Service for Supervision in the Sphere of Communications, Information Technology and Mass Communications. Registration Certificate: ПИ № ФС 77 – 53970, 30 April 2013 Impact factor of the journal «The Way of Science» – 0.543 (Global Impact Factor, Australia) EDITORIAL STAFF: Head editor: Musienko Sergey Aleksandrovich Executive editor: Manotskova Nadezhda Vasilyevna Borovik Vitaly Vitalyevich, Candidate of Technical Sciences Zharikov Valery Viktorovich, Candidate of Technical Sciences, Doctor of Economic Sciences Al-Ababneh Hasan Ali, PhD in Engineering Authors have responsibility for credibility of information set out in the articles. Editorial opinion can be out of phase with opinion of the authors. Address: Russia, Volgograd, Angarskaya St., 17 «G» E-mail: [email protected] Website: www.scienceway.ru Founder and publisher: Publishing House «Scientific survey» © Publishing House «Scientific survey», 2017 2 ISSN 2311-2158. The Way of Science. 2017. № 11 (45). УДК 53:51+54+57+631+93:902+330+101+80+340+371+32 ББК 72 Путь науки Международный научный журнал, № 11 (45), 2017 Журнал основан в 2014 г. (март) ISSN 2311-2158 Журнал выходит 12 раз в год Журнал зарегистрирован Федеральной службой по надзору в сфере связи, информационных технологий и массовых коммуникаций. -

Table of Contents
WELCOME TO LOST HORIZONS 2017 CATALOGUE Table of Contents Welcome to Lost Horizons . .15 . Great Plants/Wonderful People . 16. Nomenclatural Notes . 16. Some History . 17. Availability . .18 . Recycle . 18 Location . 18 Hours . 19 Note on Hardiness . 19. Gift Certificates . 19. Lost Horizons Garden Design, Consultation, and Construction . 20. Understanding the catalogue . 20. References . 21. Catalogue . 23. Perennials . .23 . Acanthus . .23 . Achillea . .23 . Aconitum . 23. Actaea . .24 . Agastache . .25 . Ajuga . 25. Alchemilla . 25. Allium . .26 . Alstroemeria . .26 . Alyssum . 27. Amsonia . 27. Androsace . .28 . Anemone . .28 . Anemonella . .29 . Anemonopsis . 29. Angelica . 30. Anthericum . .30 . For more info go to www.losthorizons.ca - Page 1 Aquilegia . 30. Arabis . .31 . Aralia . 31. Arenaria . 31. Arisaema . .32 . Arisarum . .33 . Armeria . .33 . Armoracia . .33 . Artemisia . 34. Arum . .34 . Aruncus . .34 . Asarum . .35 . Asclepias . .35 . Asparagus . .35 . Asphodeline . 36. Asphodelus . .36 . Aster . .36 . Astilbe . .37 . Astilboides . 37. Astragalus . .38 . Astrantia . .38 . Aubrieta . 38. Baptisia . .39 . Begonia . .39 . Bergenia . 40. Berkheya . .40 . Bletilla . 41. Boehmeria . .41 . Bolax . .41 . Brunnera . .42 . Buphthalmum . .42 . Cacalia . 43. For more info go to www.losthorizons.ca - Page 2 Caltha . 43. Campanula . 43. Cardamine . .44 . Cardiocrinum . 45. Caryopteris(Perennial) . 45. Cassia . 45. Caulophyllum . .46 . Centaurea . 46. Cephalaria . .46 . Chelone . .47 . Chelonopsis . 47. Chiastophyllum . .. -

Alfred Russel Wallace: Letters and Reminiscences, Vol
Alfred Russel Wallace: Letters and Reminiscences, Vol. 2 1 Alfred Russel Wallace: Letters and Reminiscences, Vol. 2 The Project Gutenberg EBook of Alfred Russel Wallace: Letters and Reminiscences Vol 2 (of 2), by James Marchant This eBook is for the use of anyone anywhere at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at www.gutenberg.net Title: Alfred Russel Wallace: Letters and Reminiscences Vol 2 (of 2) Author: James Marchant Release Date: June 7, 2005 [EBook #15998] Language: English Character set encoding: ISO-8859-1 *** START OF THIS PROJECT GUTENBERG EBOOK ALFRED RUSSEL WALLACE: *** Produced by Digital & Multimedia Center, Michigan State University Libraries., Marilynda Fraser-Cunliffe, Josephine Paolucci, Joshua Hutchinson and the Online Distributed Proofreading Team at http://www.pgdp.net [Transcriber's note: Footnotes moved to end of book] ALFRED RUSSEL WALLACE LETTERS AND REMINISCENCES PART III 2 [Illustration: A.R. WALLACE (1913)] Alfred Russel Wallace Letters and Reminiscences By James Marchant _With Two Photogravures and Eight Half-tone Plates_ IN TWO VOLUMES Volume II CASSELL AND COMPANY, LTD London, New York, Toronto and Melbourne 1916 CONTENTS OF VOLUME II PART III I. WALLACE'S WORKS ON BIOLOGY AND GEOGRAPHICAL DISTRIBUTION II. CORRESPONDENCE ON BIOLOGY, GEOGRAPHICAL DISTRIBUTION, ETC. (1864-98) III. CORRESPONDENCE ON BIOLOGY, GEOGRAPHICAL DISTRIBUTION, ETC. (1894-1913) PART IV 3 PART IV HOME LIFE PART V SOCIAL AND POLITICAL VIEWS PART VI SOME FURTHER PROBLEMS I. ASTRONOMY II. SPIRITUALISM PART VII CHARACTERISTICS APPENDIX: LISTS OF WALLACE'S WRITINGS INDEX LIST OF PLATES IN VOLUME II A.R.