Prevalence of Active Trachoma and Associated Risk Factors Among
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Livestock and Livestock Systems in the Bale Mountains Ecoregion
LIVESTOCK AND LIVESTOCK SYSTEMS IN THE BALE MOUNTAINS ECOREGION Fiona Flintan, Worku Chibsa, Dida Wako and Andrew Ridgewell A report for the Bale EcoRegion Sustainable Management Project, SOS Sahel Ethiopia and FARM Africa June 2008 Addis Ababa Photo: A respondent mapping grazing routes in Bale Mountains EXECUTIVE SUMMARY Livestock has been an integral part of the Bale landscape for many centuries. Until relatively recently the livestock system was extensive with small numbers of people and livestock moving in a free and mobile manner. However since the time of Haile Selassie there have been numerous influencing factors that have changed the face of livestock production in Bale. This began with the introduction of land measurement and taxes which encouraged settled agricultural expansion, aggravated by the declaration of grazing lands as ‘no-man’s lands’. At the same time large scale mechanised farms were established in the lower areas, forcing livestock producers into the higher altitude regions. More recently villagisation and resettlement programmes have promoted settlement and an increased population. However, the largest single ‘loss’ of pastoral resources occurred with the establishment of the Bale Mountains National Park (BMNP) in 1970 encompassing an area of 2400km2. This was created without the consent or even the knowledge of local resource users. The historical development of BMNP has been aimed principally, albeit intermittently, at preserving the environment as a ‘wilderness’ area by excluding habitation and customary natural resource management practices. During the former Dergue regime (1974-1991) state authority over the Park was at its strongest resulting in the forced removal of settlements and the effective colonisation of the mountain landscape. -

ETHIOPIA Humanitarian Access Situation Report June – July 2019
ETHIOPIA Humanitarian Access Situation Report June – July 2019 This report is produced by OCHA Ethiopia in collaboration with humanitarian partners. It covers the period June - July 2019. The next report will be issued around September - October 2019. OVERVIEW IUS • In June - July, Ethiopia experienced an at- TIGRAY 276 Access incidents reported tempted government overthrow in Amhara, Western socio-political unrest in Sidama (SNNPR), North Gondar Wag Hamra Central Gondar and a rise in security incidents in Southwest- Zone 4 (Fantana Rasu) AFAR ern Oromia and Gambella. The quality of ac- Zone 1 (Awsi Rasu) cess declined, limiting assistance to people AMHARA No. o incidents by one South Wello Metekel in need, against a backdrop of massive gov- Oromia East Gojam BENISHANGUL Zone 5 (Hari Rasu) 4 13 35 49 AsosaGUMUZ Siti ernment-led returns of IDP to areas of origin. Zone 3 (Gabi Rasu) North Shewa(O) North Shewa(A) Kemashi Dire Dawa urban West Wellega East Wellega DIRE DAWA West Shewa Fafan • Hostilities between Ethiopian Defense Forc- ADDIS ABABA Kelem Wellega East Hararge Finfine Special West Hararge es (EDF) and Unidentified Armed Groups Buno Bedele East Shewa Etang Special Ilu Aba Bora Jarar OROMIA Erer (UAGs) as well as inter-ethnic, remained the GAMBELA Jimma Agnewak main access obstacle, with 197 incidents Doolo Nogob West Arsi SOMALI (out of 276), mostly in Southwestern Oromia SNNP Sidama Bale Korahe (110). The Wellegas, West Guji (Oromia), and Gedeo Shabelle Gambella, were the most insecure areas for Segen Area P. West Guji Guji aid workers. Liban Borena • In June, conflict in the Wellegas scaled up, Daawa with explosive devices attacks causing ci- Source: Access Incidents database vilian casualties in urban centres. -

Prioritization of Shelter/NFI Needs
Prioritization of Shelter/NFI needs Date: 31st May 2018 Shelter and NFI Needs As of 18 May 2018, the overall number of displaced people is 345,000 households. This figure is based on DTM round 10, partner’s assessments, government requests, as well as the total of HH supported since July 2017. The S/NFI updated its prioritisation in early May and SNFI Cluster partners agreed on several criteria to guide prioritisation which include: - 1) type of emergency, 2) duration of displacement, and 3) sub-standard shelter conditions including IDPS hosted in collective centres and open-air sites and 4) % of vulnerable HH at IDP sites. Thresholds for the criteria were also agreed and in the subsequent analysis the cluster identified 193 IDP hosting woredas mostly in Oromia and Somali regions, as well as Tigray, Gambella and Addis Ababa municipality. A total of 261,830 HH are in need of urgent shelter and NFI assistance. At present the Cluster has a total of 57,000 kits in stocks and pipeline. The Cluster requires urgent funding to address the needs of 204,830 HHs that are living in desperate displacement conditions across the country. This caseload is predicted to increase as the flooding continues in the coming months. Shelter and NFI Priority Activities In terms of priority activities, the SNFI Cluster is in need of ES/NFI support for 140,259 HH displaced mainly due to flood and conflict under Pillar 2, primarily in Oromia and Somali Regions. In addition, the Shelter and NFI Cluster requires immediate funding for recovery activities to support 14,000 HH (8,000 rebuild and 6,000 repair) with transitional shelter support and shelter repair activities under Pillar 3. -

English-Full (0.5
Enhancing the Role of Forestry in Building Climate Resilient Green Economy in Ethiopia Strategy for scaling up effective forest management practices in Amhara National Regional State with particular emphasis on smallholder plantations Wubalem Tadesse Alemu Gezahegne Teshome Tesema Bitew Shibabaw Berihun Tefera Habtemariam Kassa Center for International Forestry Research Ethiopia Office Addis Ababa October 2015 Copyright © Center for International Forestry Research, 2015 Cover photo by authors FOREWORD This regional strategy document for scaling up effective forest management practices in Amhara National Regional State, with particular emphasis on smallholder plantations, was produced as one of the outputs of a project entitled “Enhancing the Role of Forestry in Ethiopia’s Climate Resilient Green Economy”, and implemented between September 2013 and August 2015. CIFOR and our ministry actively collaborated in the planning and implementation of the project, which involved over 25 senior experts drawn from Federal ministries, regional bureaus, Federal and regional research institutes, and from Wondo Genet College of Forestry and Natural Resources and other universities. The senior experts were organised into five teams, which set out to identify effective forest management practices, and enabling conditions for scaling them up, with the aim of significantly enhancing the role of forests in building a climate resilient green economy in Ethiopia. The five forest management practices studied were: the establishment and management of area exclosures; the management of plantation forests; Participatory Forest Management (PFM); agroforestry (AF); and the management of dry forests and woodlands. Each team focused on only one of the five forest management practices, and concentrated its study in one regional state. -

Ethiopia: Amhara Region Administrative Map (As of 05 Jan 2015)
Ethiopia: Amhara region administrative map (as of 05 Jan 2015) ! ! ! ! ! ! ! ! ! ! Abrha jara ! Tselemt !Adi Arikay Town ! Addi Arekay ! Zarima Town !Kerakr ! ! T!IGRAY Tsegede ! ! Mirab Armacho Beyeda ! Debark ! Debarq Town ! Dil Yibza Town ! ! Weken Town Abergele Tach Armacho ! Sanja Town Mekane Berhan Town ! Dabat DabatTown ! Metema Town ! Janamora ! Masero Denb Town ! Sahla ! Kokit Town Gedebge Town SUDAN ! ! Wegera ! Genda Wuha Town Ziquala ! Amba Giorges Town Tsitsika Town ! ! ! ! Metema Lay ArmachoTikil Dingay Town ! Wag Himra North Gonder ! Sekota Sekota ! Shinfa Tomn Negade Bahr ! ! Gondar Chilga Aukel Ketema ! ! Ayimba Town East Belesa Seraba ! Hamusit ! ! West Belesa ! ! ARIBAYA TOWN Gonder Zuria ! Koladiba Town AMED WERK TOWN ! Dehana ! Dagoma ! Dembia Maksegnit ! Gwehala ! ! Chuahit Town ! ! ! Salya Town Gaz Gibla ! Infranz Gorgora Town ! ! Quara Gelegu Town Takusa Dalga Town ! ! Ebenat Kobo Town Adis Zemen Town Bugna ! ! ! Ambo Meda TownEbinat ! ! Yafiga Town Kobo ! Gidan Libo Kemkem ! Esey Debr Lake Tana Lalibela Town Gomenge ! Lasta ! Muja Town Robit ! ! ! Dengel Ber Gobye Town Shahura ! ! ! Wereta Town Kulmesk Town Alfa ! Amedber Town ! ! KUNIZILA TOWN ! Debre Tabor North Wollo ! Hara Town Fogera Lay Gayint Weldiya ! Farta ! Gasay! Town Meket ! Hamusit Ketrma ! ! Filahit Town Guba Lafto ! AFAR South Gonder Sal!i Town Nefas mewicha Town ! ! Fendiqa Town Zege Town Anibesema Jawi ! ! ! MersaTown Semen Achefer ! Arib Gebeya YISMALA TOWN ! Este Town Arb Gegeya Town Kon Town ! ! ! ! Wegel tena Town Habru ! Fendka Town Dera -
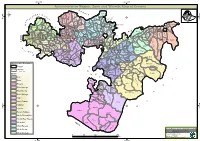
Administrative Region, Zone and Woreda Map of Oromia a M Tigray a Afar M H U Amhara a Uz N M
35°0'0"E 40°0'0"E Administrative Region, Zone and Woreda Map of Oromia A m Tigray A Afar m h u Amhara a uz N m Dera u N u u G " / m r B u l t Dire Dawa " r a e 0 g G n Hareri 0 ' r u u Addis Ababa ' n i H a 0 Gambela m s Somali 0 ° b a K Oromia Ü a I ° o A Hidabu 0 u Wara o r a n SNNPR 0 h a b s o a 1 u r Abote r z 1 d Jarte a Jarso a b s a b i m J i i L i b K Jardega e r L S u G i g n o G A a e m e r b r a u / K e t m uyu D b e n i u l u o Abay B M G i Ginde e a r n L e o e D l o Chomen e M K Beret a a Abe r s Chinaksen B H e t h Yaya Abichuna Gne'a r a c Nejo Dongoro t u Kombolcha a o Gulele R W Gudetu Kondole b Jimma Genete ru J u Adda a a Boji Dirmeji a d o Jida Goro Gutu i Jarso t Gu J o Kembibit b a g B d e Berga l Kersa Bila Seyo e i l t S d D e a i l u u r b Gursum G i e M Haro Maya B b u B o Boji Chekorsa a l d Lalo Asabi g Jimma Rare Mida M Aleltu a D G e e i o u e u Kurfa Chele t r i r Mieso m s Kegn r Gobu Seyo Ifata A f o F a S Ayira Guliso e Tulo b u S e G j a e i S n Gawo Kebe h i a r a Bako F o d G a l e i r y E l i Ambo i Chiro Zuria r Wayu e e e i l d Gaji Tibe d lm a a s Diga e Toke n Jimma Horo Zuria s e Dale Wabera n a w Tuka B Haru h e N Gimbichu t Kutaye e Yubdo W B Chwaka C a Goba Koricha a Leka a Gidami Boneya Boshe D M A Dale Sadi l Gemechis J I e Sayo Nole Dulecha lu k Nole Kaba i Tikur Alem o l D Lalo Kile Wama Hagalo o b r Yama Logi Welel Akaki a a a Enchini i Dawo ' b Meko n Gena e U Anchar a Midega Tola h a G Dabo a t t M Babile o Jimma Nunu c W e H l d m i K S i s a Kersana o f Hana Arjo D n Becho A o t -

Ethiopia: Administrative Map (August 2017)
Ethiopia: Administrative map (August 2017) ERITREA National capital P Erob Tahtay Adiyabo Regional capital Gulomekeda Laelay Adiyabo Mereb Leke Ahferom Red Sea Humera Adigrat ! ! Dalul ! Adwa Ganta Afeshum Aksum Saesie Tsaedaemba Shire Indasilase ! Zonal Capital ! North West TigrayTahtay KoraroTahtay Maychew Eastern Tigray Kafta Humera Laelay Maychew Werei Leke TIGRAY Asgede Tsimbila Central Tigray Hawzen Medebay Zana Koneba Naeder Adet Berahile Region boundary Atsbi Wenberta Western Tigray Kelete Awelallo Welkait Kola Temben Tselemti Degua Temben Mekele Zone boundary Tanqua Abergele P Zone 2 (Kilbet Rasu) Tsegede Tselemt Mekele Town Special Enderta Afdera Addi Arekay South East Ab Ala Tsegede Mirab Armacho Beyeda Woreda boundary Debark Erebti SUDAN Hintalo Wejirat Saharti Samre Tach Armacho Abergele Sanja ! Dabat Janamora Megale Bidu Alaje Sahla Addis Ababa Ziquala Maychew ! Wegera Metema Lay Armacho Wag Himra Endamehoni Raya Azebo North Gondar Gonder ! Sekota Teru Afar Chilga Southern Tigray Gonder City Adm. Yalo East Belesa Ofla West Belesa Kurri Dehana Dembia Gonder Zuria Alamata Gaz Gibla Zone 4 (Fantana Rasu ) Elidar Amhara Gelegu Quara ! Takusa Ebenat Gulina Bugna Awra Libo Kemkem Kobo Gidan Lasta Benishangul Gumuz North Wello AFAR Alfa Zone 1(Awsi Rasu) Debre Tabor Ewa ! Fogera Farta Lay Gayint Semera Meket Guba Lafto DPubti DJIBOUTI Jawi South Gondar Dire Dawa Semen Achefer East Esite Chifra Bahir Dar Wadla Delanta Habru Asayita P Tach Gayint ! Bahir Dar City Adm. Aysaita Guba AMHARA Dera Ambasel Debub Achefer Bahirdar Zuria Dawunt Worebabu Gambela Dangura West Esite Gulf of Aden Mecha Adaa'r Mile Pawe Special Simada Thehulederie Kutaber Dangila Yilmana Densa Afambo Mekdela Tenta Awi Dessie Bati Hulet Ej Enese ! Hareri Sayint Dessie City Adm. -

AFET-22 Appeal Target: US$ 27,030,875 Balance Requested from ACT Network: US$ 26,995,619
150 route de Ferney, P.O. Box 2100 1211 Geneva 2, Switzerland Appeal Tel: 41 22 791 6033 Fax: 41 22 791 6506 e-mail: [email protected] Ethiopia Coordinating Office Assistance to Drought Affected - AFET-22 Appeal Target: US$ 27,030,875 Balance Requested from ACT Network: US$ 26,995,619 Geneva, 21 November 2002 Dear Colleagues, According to the report released in September 2002 by the Ethiopia Federal Disaster Preparedness and Prevention Commission (DPPC), over 6.8 million people in Ethiopia are facing severe food shortages during the current period, with the numbers expected to rise to about 10 million people in the early part of 2003. The Ethiopian Prime Minister, Meles Zenawi was quoted earlier this month as saying that the country faces a famine worse than that of 1984 which killed nearly one million people and sparked off huge international relief efforts. He was further quoted “if the 1984 famine was a nightmare, then this will be too ghastly to contemplate”. Whether this statement could be considered accurate or not, the truth of the matter is that Ethiopia is once more faced with a very severe food crisis which calls for the international community to intervene with massive food aid. As a country highly dependent on rain fed agriculture, this years’ partial or total failure of the belg (short rains) in many areas aggravated the problem of food insecurity. The meher rains (longer rains) also came late and were erratic affecting the long cycle crops and the availability of pasture and water supplies in many pastoral areas. -

AMHARA Demography and Health
1 AMHARA Demography and Health Aynalem Adugna January 1, 2021 www.EthioDemographyAndHealth.Org 2 Amhara Suggested citation: Amhara: Demography and Health Aynalem Adugna January 1, 20201 www.EthioDemographyAndHealth.Org Landforms, Climate and Economy Located in northwestern Ethiopia the Amhara Region between 9°20' and 14°20' North latitude and 36° 20' and 40° 20' East longitude the Amhara Region has an estimated land area of about 170000 square kilometers . The region borders Tigray in the North, Afar in the East, Oromiya in the South, Benishangul-Gumiz in the Southwest and the country of Sudan to the west [1]. Amhara is divided into 11 zones, and 140 Weredas (see map at the bottom of this page). There are about 3429 kebeles (the smallest administrative units) [1]. "Decision-making power has recently been decentralized to Weredas and thus the Weredas are responsible for all development activities in their areas." The 11 administrative zones are: North Gonder, South Gonder, West Gojjam, East Gojjam, Awie, Wag Hemra, North Wollo, South Wollo, Oromia, North Shewa and Bahir Dar City special zone. [1] The historic Amhara Region contains much of the highland plateaus above 1500 meters with rugged formations, gorges and valleys, and millions of settlements for Amhara villages surrounded by subsistence farms and grazing fields. In this Region are located, the world- renowned Nile River and its source, Lake Tana, as well as historic sites including Gonder, and Lalibela. "Interspersed on the landscape are higher mountain ranges and cratered cones, the highest of which, at 4,620 meters, is Ras Dashen Terara northeast of Gonder. -

Periodic Monitoring Report Working 2016 Humanitarian Requirements Document – Ethiopia Group
DRMTechnical Periodic Monitoring Report Working 2016 Humanitarian Requirements Document – Ethiopia Group Covering 1 Jan to 31 Dec 2016 Prepared by Clusters and NDRMC Introduction The El Niño global climactic event significantly affected the 2015 meher/summer rains on the heels of failed belg/ spring rains in 2015, driving food insecurity, malnutrition and serious water shortages in many parts of the country. The Government and humanitarian partners issued a joint 2016 Humanitarian Requirements Document (HRD) in December 2015 requesting US$1.4 billion to assist 10.2 million people with food, health and nutrition, water, agriculture, shelter and non-food items, protection and emergency education responses. Following the delay and erratic performance of the belg/spring rains in 2016, a Prioritization Statement was issued in May 2016 with updated humanitarian requirements in nutrition (MAM), agriculture, shelter and non-food items and education.The Mid-Year Review of the HRD identified 9.7 million beneficiaries and updated the funding requirements to $1.2 billion. The 2016 HRD is 69 per cent funded, with contributions of $1.08 billion from international donors and the Government of Ethiopia (including carry-over resources from 2015). Under the leadership of the Government of Ethiopia delivery of life-saving and life- sustaining humanitarian assistance continues across the sectors. However, effective humanitarian response was challenged by shortage of resources, limited logistical capacities and associated delays, and weak real-time information management. This Periodic Monitoring Report (PMR) provides a summary of the cluster financial inputs against outputs and achievements against cluster objectives using secured funding since the launch of the 2016 HRD. -

View Full Text-PDF
Int.J.Curr.Res.Aca.Rev.2018; 6(10): 16-22 International Journal of Current Research and Academic Review ISSN: 2347-3215 (Online) Volume 6 Number 10 (October-2018) Journal homepage: http://www.ijcrar.com doi: https://doi.org/10.20546/ijcrar.2018.610.003 Determinants of Adoption of Teff Row Planting Practice: The Case of Baso Liben Woreda, East Gojjam Zone, Amhara Region, Ethopia Mekidelawit Ayal*, Rahmeto Negash and Agidew Abebe Department of Rural Development and Agricultural Extension, College of Agricultural Sciences, Arba Minch University, Arba Minch Ethiopia *Corresponding author Abstract Article Info The factors that affect adoption of row planting method in teff production in the area is not Accepted: 04 September 2018 studied and documented. Therefore, the main aim of this study is to assess the status of adoption Available Online: 20 October 2018 of Teff row planting technique and its determinants as well as to assess the yield difference between row planting and broadcasting practice on Teff grain production. Descriptive cross- sectional study design was used and data were collected from 147 households. Three kebeles in Keywords the woreda namely: Yelemelemem, Yeduge and Yegelawu were selected purposively. Structured Adoption; Row planting; interview schedule, key informant interview, and focus group discussion were used to collect the BasoLiben; Teff data. The data were checked for completeness, coded, entered, and analyzed using SPSS version 20.0. Descriptive and inferential statistical technique such as chi-square test and t-test were also used. Moreover, binary logistic regression analysis was used to identify determinants of adoption of Teff row planting practice. -

Urgent Need for Integrated Emergency Response in 141 Outbreak and Undernutrition Affected Woredas of Ethiopia in 2020
JOINT INTER-CLUSTER ADVOCACY PAPER URGENT NEED FOR INTEGRATED EMERGENCY RESPONSE IN 141 OUTBREAK AND UNDERNUTRITION AFFECTED WOREDAS OF ETHIOPIA IN 2020 Ethiopian population is currently facing new crises while past crises are not yet resolved. The recurrent food & undernutrition crises and increased frequency of measles and cholera outbreaks in the course of 2019 are now further exacerbated by desert locust infestations and upcoming COVID-19 pandemic. The complexification of the emergency needs requires to bring together our integrated efforts and expertise to address them. Ethiopia Health Cluster, WASH Cluster and Emergency Nutrition Coordination Unit established Health, WASH and Nutrition Technical Woking Group (HWN TWG) to further boost joint response in 141 woreda and IDP camps of Ethiopia by implementing minimum multi-sectoral response package. OUR ASKS • The HWN TWG asks the EHCT to support our initiative by providing more visibility and attention to this integrated initiative and request an opportunity to provide periodic updates at EHCT meetings. • The HWN TWG asks the Government of Ethiopia, especially NDRMC, MOH, EPHI and MOWIE, to improve the joint Health, WASH and Nutrition collaboration, to foster joint system strengthening and data sharing with humanitarian actors. • The HWN TWG asks Donors, including EHF and all the Rapid Response Mechanisms, to prioritise multisectoral funding in priority woredas and consider the multi-sectoral approach as an example of good practice. • The HWN TWG asks implementing partners to include the pilot of the Health WASH and Nutrition minimum package in their future proposals and project design. Partners are encouraged to work as a consortium to jointly implement the full package.