Original Article Bovine Trypanosomosis in Three Districts Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

(2) 185-194 Chemical Composition, Mineral Profile and Sensory
East African Journal of Sciences (2019) Volume 13 (2) 185-194 Chemical Composition, Mineral Profile and Sensory Properties of Traditional Cheese Varieties in selected areas of Eastern Gojjam, Ethiopia Mitiku Eshetu1* and Aleme Asresie2 1School of Animal and Range Sciences, Haramaya University, P.O. Box 138, Dire Dawa, Ethiopia 2Department of Animal Production and Technology, Adigrat University, Adigrat, Ethiopia Abstract: This study was conducted to evaluate the chemical composition, mineral profile and sensory properties of Metata, Ayib and Hazo traditional cheese varieties in selected areas of Eastern Gojjam. The chemical composition and mineral content of the cheese varieties were analyzed following standard procedures. Sensory analysis was also conducted by consumer panelists to assess taste, aroma, color, texture and overall acceptability of these traditional cheese varieties. Metata cheese samples had significantly (P<0.05) lower moisture content and higher titratable acidity than Ayib and Hazo cheese samples. The protein, ash, fat contents of Metata cheese samples were significantly (P<0.05) higher than Ayib and Hazo cheese samples. Moreover, phosphorus, calcium, magnesium, sodium and potassium contents of Metata cheese samples were significantly (P<0.05) higher than that of Ayib and Hazo cheese samples. Metata cheese samples had also the highest consumer acceptability scores compared to Ayib and Hazo cheese samples. In general, the results of this work showed that Metata cheese has higher nutritional value and overall sensory acceptability. -

Determinants of Rural Household's Livelihood Strategies in Machakel Woreda, East Gojjam Zone, Amhara Nation Regional State, Ethiopia
Developing Country Studies www.iiste.org ISSN 2224-607X (Paper) ISSN 2225-0565 (Online) Vol.8, No.10, 2018 Determinants of Rural Household's Livelihood Strategies in Machakel Woreda, East Gojjam Zone, Amhara Nation Regional State, Ethiopia Adey Belete Department of Disaster Risk Management & Sustainable Development, Institute of Disaster Risk Management & Food Security Studies, Bahir Dar University, P.O.Box 5501 Abstract Rural farm households face an increasing need of looking for alternative income sources to supplement their small scale agricultural activities. However, livelihood strategy is determined by complex and yet empirically untested factors in Machakel Woreda. Thus, the aim of this study was to assess the determinants of livelihood strategies in the study area. The data were obtained from 144 sample household heads that were selected through a combination of multi-stage sampling like purposive and simple random sampling techniques. Data were collected through key informant interview, focus group discussion and interview schedule. Multinomial logistic regression model used to analyze determinants of livelihood strategies. Data analysis revealed that farm alone activities has a leading contribution to the total income of sample households (69.8%) followed by non-farm activities (17.2%) and off- farm activities (13 %.). Crop production was the dominant livelihood in the study area and land fragmentation, decline in productivity, occurrence of disaster risk like crop and livestock disease, hail storm, flash flood etc. and market fluctuation were major threatens of livelihood. Four livelihood strategies namely farm alone, farm plus non-farm, farm plus off-farm and farm plus non-farm plus off-farm were identified. Age, education level, sex of household head, marital status, credit access, farm land size, livestock holding size , agro-ecology, family size, frequency of extension contact, distance from market and total net income were major determinants of livelihood strategies in the study area. -

Versus Routine Care on Improving Utilization Of
Andargie et al. Trials (2020) 21:151 https://doi.org/10.1186/s13063-019-4002-3 STUDY PROTOCOL Open Access Effectiveness of Checklist-Based Box System Interventions (CBBSI) versus routine care on improving utilization of maternal health services in Northwest Ethiopia: study protocol for a cluster randomized controlled trial Netsanet Belete Andargie1,2* , Mulusew Gerbaba Jebena2 and Gurmesa Tura Debelew2 Abstract Background: Maternal mortality is still high in Ethiopia. Antenatal care, the use of skilled delivery and postnatal care are key maternal health care services that can significantly reduce maternal mortality. However, in low- and middle-income countries, including Ethiopia, utilization of these key services is limited, and preventive, promotive and curative services are not provided as per the recommendations. The aim of this study is to examine the effectiveness of checklist-based box system interventions on improving maternal health service utilization. Methods: A community-level, cluster-randomized controlled trial will be conducted to compare the effectiveness of checklist-based box system interventions over the routine standard of care as a control arm. The intervention will use a health-extension program provided by health extension workers and midwives using a special type of health education scheduling box placed at health posts and a service utilization monitoring box placed at health centers. For this, 1200 pregnant mothers at below 16 weeks of gestation will be recruited from 30 clusters. Suspected pregnant mothers will be identified through a community survey and linked to the nearby health center. With effective communication between health centers and health posts, dropout-tracing mechanisms are implemented to help mothers resume service utilization. -

Food Insecurity Among Households with and Without Podoconiosis in East and West Gojjam, Ethiopia
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Sussex Research Online Food insecurity among households with and without podoconiosis in East and West Gojjam, Ethiopia Article (Published Version) Ketema, Kassahun, Tsegay, Girmay, Gedle, Dereje, Davey, Gail and Deribe, Kebede (2018) Food insecurity among households with and without podoconiosis in East and West Gojjam, Ethiopia. EC Nutrition, 13 (7). pp. 414-423. ISSN 2453-188X This version is available from Sussex Research Online: http://sro.sussex.ac.uk/id/eprint/76681/ This document is made available in accordance with publisher policies and may differ from the published version or from the version of record. If you wish to cite this item you are advised to consult the publisher’s version. Please see the URL above for details on accessing the published version. Copyright and reuse: Sussex Research Online is a digital repository of the research output of the University. Copyright and all moral rights to the version of the paper presented here belong to the individual author(s) and/or other copyright owners. To the extent reasonable and practicable, the material made available in SRO has been checked for eligibility before being made available. Copies of full text items generally can be reproduced, displayed or performed and given to third parties in any format or medium for personal research or study, educational, or not-for-profit purposes without prior permission or charge, provided that the authors, title and full bibliographic details are credited, a hyperlink and/or URL is given for the original metadata page and the content is not changed in any way. -
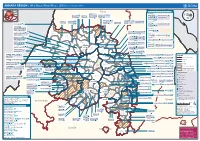
AMHARA REGION : Who Does What Where (3W) (As of 13 February 2013)
AMHARA REGION : Who Does What Where (3W) (as of 13 February 2013) Tigray Tigray Interventions/Projects at Woreda Level Afar Amhara ERCS: Lay Gayint: Beneshangul Gumu / Dire Dawa Plan Int.: Addis Ababa Hareri Save the fk Save the Save the df d/k/ CARE:f k Save the Children:f Gambela Save the Oromia Children: Children:f Children: Somali FHI: Welthungerhilfe: SNNPR j j Children:l lf/k / Oxfam GB:af ACF: ACF: Save the Save the af/k af/k Save the df Save the Save the Tach Gayint: Children:f Children: Children:fj Children:l Children: l FHI:l/k MSF Holand:f/ ! kj CARE: k Save the Children:f ! FHI:lf/k Oxfam GB: a Tselemt Save the Childrenf: j Addi Dessie Zuria: WVE: Arekay dlfk Tsegede ! Beyeda Concern:î l/ Mirab ! Concern:/ Welthungerhilfe:k Save the Children: Armacho f/k Debark Save the Children:fj Kelela: Welthungerhilfe: ! / Tach Abergele CRS: ak Save the Children:fj ! Armacho ! FHI: Save the l/k Save thef Dabat Janamora Legambo: Children:dfkj Children: ! Plan Int.:d/ j WVE: Concern: GOAL: Save the Children: dlfk Sahla k/ a / f ! ! Save the ! Lay Metema North Ziquala Children:fkj Armacho Wegera ACF: Save the Children: Tenta: ! k f Gonder ! Wag WVE: Plan Int.: / Concern: Save the dlfk Himra d k/ a WVE: ! Children: f Sekota GOAL: dlf Save the Children: Concern: Save the / ! Save: f/k Chilga ! a/ j East Children:f West ! Belesa FHI:l Save the Children:/ /k ! Gonder Belesa Dehana ! CRS: Welthungerhilfe:/ Dembia Zuria ! î Save thedf Gaz GOAL: Children: Quara ! / j CARE: WVE: Gibla ! l ! Save the Children: Welthungerhilfe: k d k/ Takusa dlfj k -

Farmers' Varietal Selection of Food Barley Genotypes in Gozamin
American-Eurasian J. Agric. & Environ. Sci., 17 (3): 232-238, 2017 ISSN 1818-6769 © IDOSI Publications, 2017 DOI: 10.5829/idosi.aejaes.2017.232.238 Farmers’ Varietal Selection of Food Barley Genotypes in Gozamin District of East Gojjam Zone, Northwestern Ethiopia Yalemtesfa Firew Guade Department of Horticulture, College of Agriculture and Natural Resources, Debre Markos University, Ethiopia Abstract: In this study, the performance of four improved food barley varieties obtained from Holleta Agricultural Research Center and local variety collected from the study area were evaluated in randomized complete block design (RCBD) with three replications under farmers’ participatory selection scheme during 2016 main cropping season in Gozamin district, East Gojjam Zone, Northwestern Ethiopia. The objectives of this study were to select adaptable and high yielding food barley genotype(s) using farmers’ preferences and to identify farmers’ preference and selection criteria of the study area. Farmers’ set; grain yield, early maturity, tillering ability and spike length as their selection criteria at maturity stage of the crop. The results of analysis of variance (ANOVA) indicated significant differences among genotypes for all traits tested except number of effective tillers and thousand seed weight which were non-significant at 5% probability level. The improved variety EH-1493 (133 days) was the earliest while local variety (142 days) was the latest. The highest mean grain yield was obtained from HB-1307 (3700 kg ha-1 ) and Cross-41/98 (3133 kg ha-1 ) whereas the lowest from the local variety (1693 kg ha 11). Similarly, HB-1307(5033 kg ha ) and Cross-41/98 (3633.33 kg ha 1) had given comparatively the highest above ground biomass yield which was used as a source of feed for animals. -

Ethiopia Round 6 SDP Questionnaire
Ethiopia Round 6 SDP Questionnaire Always 001a. Your name: [NAME] Is this your name? ◯ Yes ◯ No 001b. Enter your name below. 001a = 0 Please record your name 002a = 0 Day: 002b. Record the correct date and time. Month: Year: ◯ TIGRAY ◯ AFAR ◯ AMHARA ◯ OROMIYA ◯ SOMALIE BENISHANGUL GUMZ 003a. Region ◯ ◯ S.N.N.P ◯ GAMBELA ◯ HARARI ◯ ADDIS ABABA ◯ DIRE DAWA filter_list=${this_country} ◯ NORTH WEST TIGRAY ◯ CENTRAL TIGRAY ◯ EASTERN TIGRAY ◯ SOUTHERN TIGRAY ◯ WESTERN TIGRAY ◯ MEKELE TOWN SPECIAL ◯ ZONE 1 ◯ ZONE 2 ◯ ZONE 3 ZONE 5 003b. Zone ◯ ◯ NORTH GONDAR ◯ SOUTH GONDAR ◯ NORTH WELLO ◯ SOUTH WELLO ◯ NORTH SHEWA ◯ EAST GOJAM ◯ WEST GOJAM ◯ WAG HIMRA ◯ AWI ◯ OROMIYA 1 ◯ BAHIR DAR SPECIAL ◯ WEST WELLEGA ◯ EAST WELLEGA ◯ ILU ABA BORA ◯ JIMMA ◯ WEST SHEWA ◯ NORTH SHEWA ◯ EAST SHEWA ◯ ARSI ◯ WEST HARARGE ◯ EAST HARARGE ◯ BALE ◯ SOUTH WEST SHEWA ◯ GUJI ◯ ADAMA SPECIAL ◯ WEST ARSI ◯ KELEM WELLEGA ◯ HORO GUDRU WELLEGA ◯ Shinile ◯ Jijiga ◯ Liben ◯ METEKEL ◯ ASOSA ◯ PAWE SPECIAL ◯ GURAGE ◯ HADIYA ◯ KEMBATA TIBARO ◯ SIDAMA ◯ GEDEO ◯ WOLAYITA ◯ SOUTH OMO ◯ SHEKA ◯ KEFA ◯ GAMO GOFA ◯ BENCH MAJI ◯ AMARO SPECIAL ◯ DAWURO ◯ SILTIE ◯ ALABA SPECIAL ◯ HAWASSA CITY ADMINISTRATION ◯ AGNEWAK ◯ MEJENGER ◯ HARARI ◯ AKAKI KALITY ◯ NEFAS SILK-LAFTO ◯ KOLFE KERANIYO 2 ◯ GULELE ◯ LIDETA ◯ KIRKOS-SUB CITY ◯ ARADA ◯ ADDIS KETEMA ◯ YEKA ◯ BOLE ◯ DIRE DAWA filter_list=${level1} ◯ TAHTAY ADIYABO ◯ MEDEBAY ZANA ◯ TSELEMTI ◯ SHIRE ENIDASILASE/TOWN/ ◯ AHIFEROM ◯ ADWA ◯ TAHTAY MAYCHEW ◯ NADER ADET ◯ DEGUA TEMBEN ◯ ABIYI ADI/TOWN/ ◯ ADWA/TOWN/ ◯ AXUM/TOWN/ ◯ SAESI TSADAMBA ◯ KLITE -

Result of Scoping (PDF/760KB)
Summary of Preparatory Study Date: February, 2011 1. Title of the Cooperation Project, Relevant Project Report Project for the Rehabilitation of the Trunk Road, Phase IV (Dejen – Debre Markos Section) 2. Categorization and Reasons Category B Major reasons for categorization are as follows: - The project is to improve the current condition of the road with paving, not new construction. - Social and natural adverse impacts will be limited or minimized by the implementation of planned mitigation measures especially if there are any protected areas or considerable habitats such as national parks or conservation areas within close proximity to the project area. - With regard to the social impacts, although some resettlements are caused, the degree of the impact is not significant and minimized by appropriate implementation of the land acquisition and resettlement action plan. - The project has been categorized as “Schedule-2 Project” which does not require the Full scale EIA on the Ethiopian EIA Law (Environmental Impact Assessment Guideline Document (2000/ Environmental Protection Authority)). It means that the project does not have significant impacts on the social and natural areas. 3. Outline of the Project (Objectives, Justification, Activites, Location, Scale, etcetera) Ninety five percent (95%) of the international cargo transportation and interurban transportation in Ethiopia is borne by road traffic. The improvement of low density of the paved road conditions (paved road length 42,429 km compared to 1,100,000 square km of land area) and the road condition (51% of the total road length is in poor condition) is a critical issue for Ethiopia. To improve this situation, Ethiopian Roads Authority (ERA) has endeavoured to develop the road network, especially the construction of the arterial roads based on the Road Sector Development Program (RSDP) from 1997 under the backing of foreign countries and international organizations. -

Farmers Willingness to Participate in Voluntary Land Consolidation in Gozamin District, Ethiopia
land Article Farmers Willingness to Participate In Voluntary Land Consolidation in Gozamin District, Ethiopia Abebaw Andarge Gedefaw 1,2,* , Clement Atzberger 1 , Walter Seher 3 and Reinfried Mansberger 1 1 Institute of Surveying, Remote Sensing and Land Information, University of Natural Resources and Life Sciences Vienna, Peter-Jordan-Strasse 82, 1190 Vienna, Austria; [email protected] (C.A.); [email protected] (R.M.) 2 Institute of Land Administration, Debre Markos University, 269 Debre Markos, Ethiopia 3 Institute of Spatial Planning, Environmental Planning and Land Rearrangement, University of Natural Resources and Life Sciences Vienna, Peter-Jordan-Strasse 82, 1190 Vienna, Austria; [email protected] * Correspondence: [email protected] Received: 10 September 2019; Accepted: 10 October 2019; Published: 12 October 2019 Abstract: In many African countries and especially in the highlands of Ethiopia—the investigation site of this paper—agricultural land is highly fragmented. Small and scattered parcels impede a necessary increase in agricultural efficiency. Land consolidation is a proper tool to solve inefficiencies in agricultural production, as it enables consolidating plots based on the consent of landholders. Its major benefits are that individual farms get larger, more compact, contiguous parcels, resulting in lower cultivation efforts. This paper investigates the determinants influencing the willingness of landholder farmers to participate in voluntary land consolidation processes. The study was conducted in Gozamin District, Amhara Region, Ethiopia. The study was mainly based on survey data collected from 343 randomly selected landholder farmers. In addition, structured interviews and focus group discussions with farmers were held. The collected data were analyzed quantitatively mainly by using a logistic regression model and qualitatively by using focus group discussions and expert panels. -

English-Full (0.5
Enhancing the Role of Forestry in Building Climate Resilient Green Economy in Ethiopia Strategy for scaling up effective forest management practices in Amhara National Regional State with particular emphasis on smallholder plantations Wubalem Tadesse Alemu Gezahegne Teshome Tesema Bitew Shibabaw Berihun Tefera Habtemariam Kassa Center for International Forestry Research Ethiopia Office Addis Ababa October 2015 Copyright © Center for International Forestry Research, 2015 Cover photo by authors FOREWORD This regional strategy document for scaling up effective forest management practices in Amhara National Regional State, with particular emphasis on smallholder plantations, was produced as one of the outputs of a project entitled “Enhancing the Role of Forestry in Ethiopia’s Climate Resilient Green Economy”, and implemented between September 2013 and August 2015. CIFOR and our ministry actively collaborated in the planning and implementation of the project, which involved over 25 senior experts drawn from Federal ministries, regional bureaus, Federal and regional research institutes, and from Wondo Genet College of Forestry and Natural Resources and other universities. The senior experts were organised into five teams, which set out to identify effective forest management practices, and enabling conditions for scaling them up, with the aim of significantly enhancing the role of forests in building a climate resilient green economy in Ethiopia. The five forest management practices studied were: the establishment and management of area exclosures; the management of plantation forests; Participatory Forest Management (PFM); agroforestry (AF); and the management of dry forests and woodlands. Each team focused on only one of the five forest management practices, and concentrated its study in one regional state. -

Ethiopia: Amhara Region Administrative Map (As of 05 Jan 2015)
Ethiopia: Amhara region administrative map (as of 05 Jan 2015) ! ! ! ! ! ! ! ! ! ! Abrha jara ! Tselemt !Adi Arikay Town ! Addi Arekay ! Zarima Town !Kerakr ! ! T!IGRAY Tsegede ! ! Mirab Armacho Beyeda ! Debark ! Debarq Town ! Dil Yibza Town ! ! Weken Town Abergele Tach Armacho ! Sanja Town Mekane Berhan Town ! Dabat DabatTown ! Metema Town ! Janamora ! Masero Denb Town ! Sahla ! Kokit Town Gedebge Town SUDAN ! ! Wegera ! Genda Wuha Town Ziquala ! Amba Giorges Town Tsitsika Town ! ! ! ! Metema Lay ArmachoTikil Dingay Town ! Wag Himra North Gonder ! Sekota Sekota ! Shinfa Tomn Negade Bahr ! ! Gondar Chilga Aukel Ketema ! ! Ayimba Town East Belesa Seraba ! Hamusit ! ! West Belesa ! ! ARIBAYA TOWN Gonder Zuria ! Koladiba Town AMED WERK TOWN ! Dehana ! Dagoma ! Dembia Maksegnit ! Gwehala ! ! Chuahit Town ! ! ! Salya Town Gaz Gibla ! Infranz Gorgora Town ! ! Quara Gelegu Town Takusa Dalga Town ! ! Ebenat Kobo Town Adis Zemen Town Bugna ! ! ! Ambo Meda TownEbinat ! ! Yafiga Town Kobo ! Gidan Libo Kemkem ! Esey Debr Lake Tana Lalibela Town Gomenge ! Lasta ! Muja Town Robit ! ! ! Dengel Ber Gobye Town Shahura ! ! ! Wereta Town Kulmesk Town Alfa ! Amedber Town ! ! KUNIZILA TOWN ! Debre Tabor North Wollo ! Hara Town Fogera Lay Gayint Weldiya ! Farta ! Gasay! Town Meket ! Hamusit Ketrma ! ! Filahit Town Guba Lafto ! AFAR South Gonder Sal!i Town Nefas mewicha Town ! ! Fendiqa Town Zege Town Anibesema Jawi ! ! ! MersaTown Semen Achefer ! Arib Gebeya YISMALA TOWN ! Este Town Arb Gegeya Town Kon Town ! ! ! ! Wegel tena Town Habru ! Fendka Town Dera