Phenotypic Characterization and Symbiotic Effectiveness Test Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(2) 185-194 Chemical Composition, Mineral Profile and Sensory
East African Journal of Sciences (2019) Volume 13 (2) 185-194 Chemical Composition, Mineral Profile and Sensory Properties of Traditional Cheese Varieties in selected areas of Eastern Gojjam, Ethiopia Mitiku Eshetu1* and Aleme Asresie2 1School of Animal and Range Sciences, Haramaya University, P.O. Box 138, Dire Dawa, Ethiopia 2Department of Animal Production and Technology, Adigrat University, Adigrat, Ethiopia Abstract: This study was conducted to evaluate the chemical composition, mineral profile and sensory properties of Metata, Ayib and Hazo traditional cheese varieties in selected areas of Eastern Gojjam. The chemical composition and mineral content of the cheese varieties were analyzed following standard procedures. Sensory analysis was also conducted by consumer panelists to assess taste, aroma, color, texture and overall acceptability of these traditional cheese varieties. Metata cheese samples had significantly (P<0.05) lower moisture content and higher titratable acidity than Ayib and Hazo cheese samples. The protein, ash, fat contents of Metata cheese samples were significantly (P<0.05) higher than Ayib and Hazo cheese samples. Moreover, phosphorus, calcium, magnesium, sodium and potassium contents of Metata cheese samples were significantly (P<0.05) higher than that of Ayib and Hazo cheese samples. Metata cheese samples had also the highest consumer acceptability scores compared to Ayib and Hazo cheese samples. In general, the results of this work showed that Metata cheese has higher nutritional value and overall sensory acceptability. -
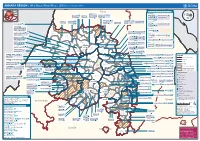
AMHARA REGION : Who Does What Where (3W) (As of 13 February 2013)
AMHARA REGION : Who Does What Where (3W) (as of 13 February 2013) Tigray Tigray Interventions/Projects at Woreda Level Afar Amhara ERCS: Lay Gayint: Beneshangul Gumu / Dire Dawa Plan Int.: Addis Ababa Hareri Save the fk Save the Save the df d/k/ CARE:f k Save the Children:f Gambela Save the Oromia Children: Children:f Children: Somali FHI: Welthungerhilfe: SNNPR j j Children:l lf/k / Oxfam GB:af ACF: ACF: Save the Save the af/k af/k Save the df Save the Save the Tach Gayint: Children:f Children: Children:fj Children:l Children: l FHI:l/k MSF Holand:f/ ! kj CARE: k Save the Children:f ! FHI:lf/k Oxfam GB: a Tselemt Save the Childrenf: j Addi Dessie Zuria: WVE: Arekay dlfk Tsegede ! Beyeda Concern:î l/ Mirab ! Concern:/ Welthungerhilfe:k Save the Children: Armacho f/k Debark Save the Children:fj Kelela: Welthungerhilfe: ! / Tach Abergele CRS: ak Save the Children:fj ! Armacho ! FHI: Save the l/k Save thef Dabat Janamora Legambo: Children:dfkj Children: ! Plan Int.:d/ j WVE: Concern: GOAL: Save the Children: dlfk Sahla k/ a / f ! ! Save the ! Lay Metema North Ziquala Children:fkj Armacho Wegera ACF: Save the Children: Tenta: ! k f Gonder ! Wag WVE: Plan Int.: / Concern: Save the dlfk Himra d k/ a WVE: ! Children: f Sekota GOAL: dlf Save the Children: Concern: Save the / ! Save: f/k Chilga ! a/ j East Children:f West ! Belesa FHI:l Save the Children:/ /k ! Gonder Belesa Dehana ! CRS: Welthungerhilfe:/ Dembia Zuria ! î Save thedf Gaz GOAL: Children: Quara ! / j CARE: WVE: Gibla ! l ! Save the Children: Welthungerhilfe: k d k/ Takusa dlfj k -

Result of Scoping (PDF/760KB)
Summary of Preparatory Study Date: February, 2011 1. Title of the Cooperation Project, Relevant Project Report Project for the Rehabilitation of the Trunk Road, Phase IV (Dejen – Debre Markos Section) 2. Categorization and Reasons Category B Major reasons for categorization are as follows: - The project is to improve the current condition of the road with paving, not new construction. - Social and natural adverse impacts will be limited or minimized by the implementation of planned mitigation measures especially if there are any protected areas or considerable habitats such as national parks or conservation areas within close proximity to the project area. - With regard to the social impacts, although some resettlements are caused, the degree of the impact is not significant and minimized by appropriate implementation of the land acquisition and resettlement action plan. - The project has been categorized as “Schedule-2 Project” which does not require the Full scale EIA on the Ethiopian EIA Law (Environmental Impact Assessment Guideline Document (2000/ Environmental Protection Authority)). It means that the project does not have significant impacts on the social and natural areas. 3. Outline of the Project (Objectives, Justification, Activites, Location, Scale, etcetera) Ninety five percent (95%) of the international cargo transportation and interurban transportation in Ethiopia is borne by road traffic. The improvement of low density of the paved road conditions (paved road length 42,429 km compared to 1,100,000 square km of land area) and the road condition (51% of the total road length is in poor condition) is a critical issue for Ethiopia. To improve this situation, Ethiopian Roads Authority (ERA) has endeavoured to develop the road network, especially the construction of the arterial roads based on the Road Sector Development Program (RSDP) from 1997 under the backing of foreign countries and international organizations. -

English-Full (0.5
Enhancing the Role of Forestry in Building Climate Resilient Green Economy in Ethiopia Strategy for scaling up effective forest management practices in Amhara National Regional State with particular emphasis on smallholder plantations Wubalem Tadesse Alemu Gezahegne Teshome Tesema Bitew Shibabaw Berihun Tefera Habtemariam Kassa Center for International Forestry Research Ethiopia Office Addis Ababa October 2015 Copyright © Center for International Forestry Research, 2015 Cover photo by authors FOREWORD This regional strategy document for scaling up effective forest management practices in Amhara National Regional State, with particular emphasis on smallholder plantations, was produced as one of the outputs of a project entitled “Enhancing the Role of Forestry in Ethiopia’s Climate Resilient Green Economy”, and implemented between September 2013 and August 2015. CIFOR and our ministry actively collaborated in the planning and implementation of the project, which involved over 25 senior experts drawn from Federal ministries, regional bureaus, Federal and regional research institutes, and from Wondo Genet College of Forestry and Natural Resources and other universities. The senior experts were organised into five teams, which set out to identify effective forest management practices, and enabling conditions for scaling them up, with the aim of significantly enhancing the role of forests in building a climate resilient green economy in Ethiopia. The five forest management practices studied were: the establishment and management of area exclosures; the management of plantation forests; Participatory Forest Management (PFM); agroforestry (AF); and the management of dry forests and woodlands. Each team focused on only one of the five forest management practices, and concentrated its study in one regional state. -

Field Visit Report of Dejen Woreda of Amhara Region
Field Visit Report of Dejen Woreda of Amhara Region Yohannes Melaku and Mussie Hailegeorgis from FTAT had a field visit to Dejen Woreda of Amhara region from October 02-05, 2017. The objectives of the field visit were to: • Assess the status of implementation of CR-WSP in the woreda, • Support woreda CR-WSP technical team in developing CR-WSP, and • Brief WWT on the developed CR-WSP and discuss on their role in supporting the CR- WSP technical team in the course of the plan developed. Status of CR-WSP implementation the woreda One of the COWASH woreda in Amhara region which has started implementing CR-WSP is Dejen Woreda, which is one of the woreda in East Gojam Zone of Amhara Region. Dejen Woreda CR-WSP team got CR-WSP training by Amhara water bureau staffs and RSU in the Month of April 2017. Having taken the training, the woreda team selected one micro- watersheds (Giraram micro-watershed in Sub-Shengo Kebele) for piloting CR-WSP in the woreda, and established the Kebele level CR-WSP teams for the micro-watersheds. The woreda team gave CR-WSP training to the Kebele teams and WASHCOs of the water points found in the micro-watershed. There are six HDWs included in the micro-watershed. Risk assessment matrix developed and improvement plan was prepared by the Woreda and Kebele CR-WSP team for the micro-watershed. They did good job in this regard. They did it as per the CR-WSP training manual. However, it was not comprehensive. They have also started implementing CR-WSP as per the plan prepared. -

Ethiopia Integrated Agro-Industrial Parks (Scpz) Support Project
Language: English Original: English PROJECT: ETHIOPIA INTEGRATED AGRO-INDUSTRIAL PARKS (SCPZ) SUPPORT PROJECT COUNTRIES: ETHIOPIA ESIA SUMMARY FOR THE 4 PROPOSED IAIPs AND RTCs LOCATED IN SOUTH WEST AMHARA REGION, CENTRAL EASTERN OROMIA REGION, WESTERN TIGRAY REGION AND EASTERN SNNP REGION, ETHIOPIA. Date: July 2018 Team Leader: C. EZEDINMA, Principal Agro Economist AHFR2 Preparation Team E&S Team Members: E.B. KAHUBIRE, Social Development Officer, RDGE4 /SNSC 1 1. INTRODUCTION 1.1. The Federal Democratic Republic of Ethiopia (FDRE) committed to a five-year undertaking, as part of the first Growth and Transformation Plan (GTP I) to build the foundation to launch the Country from a predominantly agrarian economy into industrialization. Among the sectors to which the second Growth and Transformation Plan (GTP II) gives emphasis is manufacturing and industrialization to provide the basis for economic structural change; and a central element in this strategy for transforming the industry sector is development and expansion of industrial parks and villages around the country. 1.2. The development of Integrated Agro Industrial Parks (IAIPs) and accompanying Rural Transformation Centres (RTCs) forms part of the government-run Industrial Parks Development Corporations (IPDC) strategy to make Ethiopia’s agricultural sector globally competitive. The concept is driven by a holistic approach to develop integrated Agro Commodity Procurement Zones (ACPZs) and IAIPs with state of-the-art infrastructure with backward and forward linkages based on the Inclusive and Sustainable Industrial Development model. The concept of IAIPs is to integrate various value chain components via the cluster approach. Associated RTCs are to act as collection points for fresh farm feed and agricultural produce to be transported to the IAIPs where the processing, management, and distributing (including export) activities are to take place. -

Dissemination, Adoption and Impacts of Multi-Crop Threshers in Ethiopia: Experiences of Sasakawa Global 2000
SASAKAWA AFRICA ASSOCIATION SASAKAWA GLOBAL 2000-ETHIOPIA SG2000 Working Paper, No. 04, 2015 Dissemination, Adoption and Impacts of Multi-Crop Threshers in Ethiopia: Experiences of Sasakawa Global 2000 Monitoring, Evaluation, Learning and Sharing Theme Sasakawa Global 2000-Ethiopia December 2015 Addis Ababa, Ethiopia SG2000 Working Paper, No. 04, 2015 Dissemination, Adoption and Impacts of Multi-Crop Threshers in Ethiopia: Experience of Sasakawa Global 2000 Multi-authored report by: Monitoring, Evaluation, Learning and Sharing (MELS) and Postharvest and Agro-processing (PHAP) Teams, Sasakawa Global 2000-Ethiopia MELS Team, Ethiopia PHAP Team Wondwossen Tsegaye* Aberash Tsehay Fikadu Chala Oumer Taha Kumsa Dandena Wondiye Gezahegn Bedaso Taye Teshome Lemma Samuel Amare** SAA Regional Office SG2000 - Ethiopia(Management) MELS Theme: Dr. Kebba Ngumbo Sima Dr. Aberra Debelo and Ethiopia Tadesse Dr. Habtu Asefa PHAP Theme: Eng. Leonides Halos-Kim Sasakawa Global 2000 Ethiopia (SG2000) Sasakawa Africa Association (SAA) Address: P.O. Box: 12771 Address: P.O. Box: 24135 code 1000 Addis Ababa, Ethiopia Addis Ababa, Ethiopia Tel. +251-116-628810/11/15 Tel. +251-116-477-777 *All correspondences to Wondwossen Tsegaye ([email protected] or [email protected]) ** A freelance consultant participated in the study undertaken in 2013 Dissemination, Adoption and Impacts of Multi-Crop Threshers in Ethiopia: Experiences of Sasakawa Global 2000 Dissemination, Adoption and Impacts of Multi-Crop Threshers in Ethiopia: Experiences of Sasakawa Global 2000 -

Rate of HIV Transmission and Associated Factors Among HIV
Moges et al. BMC Infectious Diseases (2017) 17:475 DOI 10.1186/s12879-017-2578-3 RESEARCH ARTICLE Open Access Rate of HIV transmission and associated factors among HIV-exposed infants in selected health facilities of East and West Gojjam Zones, Northwest Ethiopia; retrospective cohort study Nurilign Abebe Moges, Getachew Mullu Kassa* and Dube Jara Boneya Abstract Background: In 2014, there were 170,000 new HIV-infected children globally. The rate of HIV transmission from mother to child in Ethiopia was 18%. Though there are a number of HIV-related studies conducted in Ethiopia, there is a scarcity of evidence on the rate of mother to child transmission. So, the aim of this study was to determine the rate of HIV transmission and associated factors among HIV-exposed infants in selected health facilities in East and West Gojjam Zones, Northwest Ethiopia. Methods: Retrospective cohort study design was conducted. A total of 305 exposed infant- and mother pairs were included in this study. Data were collected from seven selected health facilities in East and West Gojjam Zone, Northwest Ethiopia. The study included a four-year duration PMTCT data, registered from July/2011 to July/2015. Data was collected using a prepared checklist. Data was entered using EpiData and analyzed using SPSS software. Descriptive, bivariate and multiple variable logistic regression analysis were conducted. A p-value less than 0.05 were used to declare statistical significant association. Result: Three hundred five infants and their mothers were included in this study. The mean age of mothers was 27. 4 with a standard deviation of 4.3 years. -

Woreda-Level Crop Production Rankings in Ethiopia: a Pooled Data Approach
Woreda-Level Crop Production Rankings in Ethiopia: A Pooled Data Approach 31 January 2015 James Warner Tim Stehulak Leulsegged Kasa International Food Policy Research Institute (IFPRI) Addis Ababa, Ethiopia INTERNATIONAL FOOD POLICY RESEARCH INSTITUTE The International Food Policy Research Institute (IFPRI) was established in 1975. IFPRI is one of 15 agricultural research centers that receive principal funding from governments, private foundations, and international and regional organizations, most of which are members of the Consultative Group on International Agricultural Research (CGIAR). RESEARCH FOR ETHIOPIA’S AGRICULTURE POLICY (REAP): ANALYTICAL SUPPORT FOR THE AGRICULTURAL TRANSFORMATION AGENCY (ATA) IFPRI gratefully acknowledges the generous financial support from the Bill and Melinda Gates Foundation (BMGF) for IFPRI REAP, a five-year project to support the Ethiopian ATA. The ATA is an innovative quasi-governmental agency with the mandate to test and evaluate various technological and institutional interventions to raise agricultural productivity, enhance market efficiency, and improve food security. REAP will support the ATA by providing research-based analysis, tracking progress, supporting strategic decision making, and documenting best practices as a global public good. DISCLAIMER This report has been prepared as an output for REAP and has not been reviewed by IFPRI’s Publication Review Committee. Any views expressed herein are those of the authors and do not necessarily reflect the policies or views of IFPRI, the Federal Reserve Bank of Cleveland, or the Board of Governors of the Federal Reserve System. AUTHORS James Warner, International Food Policy Research Institute Research Coordinator, Markets, Trade and Institutions Division, Addis Ababa, Ethiopia [email protected] Timothy Stehulak, Federal Reserve Bank of Cleveland Research Analyst, P.O. -

Undiagnosed Diabetes Mellitus and Related Factors in East Gojjam (NW Ethiopia) in 2016: a Community-Based Study
Journal of Public Health Research 2017; volume 6:834 Article Undiagnosed diabetes mellitus and related factors in East Gojjam (NW Ethiopia) in 2016: a community-based study Amsalu Taye Wondemagegn,1 Habtamu Mellie Bizuayehu,2 Dagninet Derebe Abie,3 Getachew Mengistu Ayalneh,4 Tenaw Yimer Tiruye,2 Mequanint Taddele Tessema2 1Department of Biomedical Science, School of Medicine, Debre Markos University; 2Department of Public Health, College of Health Sciences, Debre Markos University; 3Department of Pharmacy, College of Medicine and Health Sciences, Bahir Dar University; 4Department of Medical Laboratory, College of Health Sciences, Debre Markos University, Ethiopia Conclusions. In conclusion, this study revealed a relatively Significance for public health high prevalence of undiagnosed DM in the study area. The occur- Currently, diabetes is the second most common non-communicable disease rence of undiagnosed DM was significantly higher when associat- in Ethiopia. Its burden is 4.8% in this country, though three quarter of its ed with the age of the participants, their marital status, history of population live with undiagnosed diabetes mellitus (DM), which could lead hypertension, diabetes family history, history of gestational dia- to several complications such as heart failure, blood vessels, eyes, kidneys, betes mellitus, current smoking practices and sedentary behaviour. and nerves damages. Evidence shows that the disease is increasing through Thus, efforts have to be made, particularly by the individuals time. Early detection of DM is vital for a timely intervention to prevent life- involved in health practice, to early detect the disease and thereby threatening complications. Efforts should be made by politicians, decision makers and other healthy institutions to implement screening modality and initiate a suitable therapeutic service, before complications arise early interventions. -

Assessment of the Veterinary Cost Recovery Scheme in the Amhara Region, Ethiopia
Woldemariam et al., Ethiop. Vet. J., 2018, 22 (1), 87-98 DOI https://dx.doi.org/10.4314/evj.v22i1.7 Ethiopian Veterinary Journal Assessment of the veterinary cost recovery scheme in the Amhara region, Ethiopia Solomon Woldemariam1*, Ali Abdi1, Wondwosen Asfaw2 and Tesfaye Haile2 1*HABCON Consult, Addis Ababa, Ethiopia 2AGP-Livestock Market Development Project, Addis Ababa, Ethiopia *corresponding author email: [email protected] Abstract This study was conducted in November, 2015 in selected Woredas of Amhara region with the aim of assessing the impact of veterinary cost recovery on ef- ficiency of public private livestock services and to share the experience of the region with other regional states. Structured questionnaire and a field survey with focus group discussion were applied on a total of 475 randomly selected households. Animal health service in the Amhara region is clearly dominated by the public sector. Private-sector involvement was prominent only in the veterinary drug sales and treatment services. Seventy five percent of livestock owners responded that they received veterinary services from their residence within 1km radius. The results indicated that only 18% of respondents were satisfied by clinical examination provided by public sector. There was wide dis- parity in the effectiveness of delivery of animal health services between public and private sectors in the study Woredas. Majority of farmers were not been satisfied with the services provided by private sector. The supply of drugs is increasing especially after implementation of veterinary cost recovery scheme but with the limited ranges. Most respondents had positive views regarding availability of veterinary drugs (62.2%) and vaccines (78.1%). -

Original Article Bovine Trypanosomosis in Three Districts Of
Original Article Bovine Trypanosomosis in three districts of East Gojjam Zone bordering the Blue Nile River in Ethiopia Adane Mihret,1,2 Gezahagne Mamo,3 1Addis Ababa University, Faculty of Medicine, Addis Ababa, Ethiopia; 2Amhara region Agriculture Bureau, Bahi Dar, Ethiopia; 3Addis Ababa University, Faculty of Veterinary Medicine, Debre Zeit, Ethiopia. Abstract Background: Bovine trypanosomosis is a serious constraint to agricultural production in extensive areas of Ethiopia. Methodology: A cross-sectional study was conducted to determine the prevalence of bovine infection with trypanosomes and to identify the prevailed trypanosome species in three districts of the East Gojjam zone bordering the Blue Nile River from March 2005 to February 2006. Cattle from 9 different localities were checked using microscopical examination of wet blood smears, thin and stained bloodsmears, and by blood centrifugation followed by the examination of the resultant buffy coats. Result: Of the total 3,360 cattle investigated, 8.2% (3.5%, 11.6% and 9.4% from Dejen, Machakel and Baso-Liben districts respectively) were found to be infected with trypanosomes. Of the total 275 positive animals, 249 (90.5%) appeared to be infected with Trypanosoma vivax ; 11 (4%) were infected with T. congolense ; and 15 (5.5%) were infected with mixed infection of T. vivax and T. congolense . The prevalence of infection with T. vivax was significantly higher than that of T. congolense (P <0.001). Taking 24-46% as normal PCV value, the mean PCV for the trypanosome-infected cattle (22.09%) was lower than those for the trypanosome-negative animals (26.03). Conclusion: Trypanosomosis is a disease of considerable importance to the major economic districts bordering the Blue Nile River of the East Gojjam zone, Ethiopia, given the disease’s potential to threaten the health and productivity of cattle in this region.