Chromosome Analysis of Bidens Polylepis and Bidens Coronata (Compositae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table of Contents
New Hampshire Natural Heritage Bureau DRED – Division of Forests & Lands PO Box 1856 – 172 Pembroke Road, Concord, NH 03302-1856 (603) 271-2215 Comparison of Alternative Wetland Assessment Methods at Five Restoration Sites in New Hampshire March 2013 A report prepared by the New Hampshire Natural Heritage Bureau DRED Division of Forests & Lands, Concord, NH Completed under EPA Grant # CD-96155401 A Quick Overview of the NH Natural Heritage Bureau's Purpose and Policies The New Hampshire Native Plant Protection Act The Natural Heritage database contains (RSA 217-A) declared that native plants should information about more than 7,000 plant, animal, be protected and conserved for human need and and natural community occurrences in New enjoyment, the interests of science, and the Hampshire. economy of the state. The state maintains and enhances populations of native plants to insure their perpetuation as viable ecosystem In addition, Natural Heritage develops and components. implements measures for the protection, conservation, enhancement, and management of native New Hampshire plants. State agencies The Natural Heritage Bureau administers the assist and cooperate with the Natural Heritage Native Plant Protection Act. Natural Heritage Bureau to carry out the purposes of the Native collects and analyzes data on the status, location, Plant Protection Act. The Natural Heritage and distribution of rare or declining native plant Bureau also assists and advises the private sector species and exemplary natural communities in upon request. the state. -

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

Complete Iowa Plant Species List
!PLANTCO FLORISTIC QUALITY ASSESSMENT TECHNIQUE: IOWA DATABASE This list has been modified from it's origional version which can be found on the following website: http://www.public.iastate.edu/~herbarium/Cofcons.xls IA CofC SCIENTIFIC NAME COMMON NAME PHYSIOGNOMY W Wet 9 Abies balsamea Balsam fir TREE FACW * ABUTILON THEOPHRASTI Buttonweed A-FORB 4 FACU- 4 Acalypha gracilens Slender three-seeded mercury A-FORB 5 UPL 3 Acalypha ostryifolia Three-seeded mercury A-FORB 5 UPL 6 Acalypha rhomboidea Three-seeded mercury A-FORB 3 FACU 0 Acalypha virginica Three-seeded mercury A-FORB 3 FACU * ACER GINNALA Amur maple TREE 5 UPL 0 Acer negundo Box elder TREE -2 FACW- 5 Acer nigrum Black maple TREE 5 UPL * Acer rubrum Red maple TREE 0 FAC 1 Acer saccharinum Silver maple TREE -3 FACW 5 Acer saccharum Sugar maple TREE 3 FACU 10 Acer spicatum Mountain maple TREE FACU* 0 Achillea millefolium lanulosa Western yarrow P-FORB 3 FACU 10 Aconitum noveboracense Northern wild monkshood P-FORB 8 Acorus calamus Sweetflag P-FORB -5 OBL 7 Actaea pachypoda White baneberry P-FORB 5 UPL 7 Actaea rubra Red baneberry P-FORB 5 UPL 7 Adiantum pedatum Northern maidenhair fern FERN 1 FAC- * ADLUMIA FUNGOSA Allegheny vine B-FORB 5 UPL 10 Adoxa moschatellina Moschatel P-FORB 0 FAC * AEGILOPS CYLINDRICA Goat grass A-GRASS 5 UPL 4 Aesculus glabra Ohio buckeye TREE -1 FAC+ * AESCULUS HIPPOCASTANUM Horse chestnut TREE 5 UPL 10 Agalinis aspera Rough false foxglove A-FORB 5 UPL 10 Agalinis gattingeri Round-stemmed false foxglove A-FORB 5 UPL 8 Agalinis paupercula False foxglove -

Additions to the New Flora of Vermont
Gilman, A.V. Additions to the New Flora of Vermont. Phytoneuron 2016-19: 1–16. Published 3 March 2016. ISSN 2153 733X ADDITIONS TO THE NEW FLORA OF VERMONT ARTHUR V. GILMAN Gilman & Briggs Environmental 1 Conti Circle, Suite 5, Barre, Vermont 05641 [email protected] ABSTRACT Twenty-two species of vascular plants are reported for the state of Vermont, additional to those reported in the recently published New Flora of Vermont. These are Agrimonia parviflora, Althaea officinalis , Aralia elata , Beckmannia syzigachne , Bidens polylepis , Botrychium spathulatum, Carex panicea , Carex rostrata, Eutrochium fistulosum , Ficaria verna, Hypopitys lanuginosa, Juncus conglomeratus, Juncus diffusissimus, Linum striatum, Lipandra polysperma , Matricaria chamomilla, Nabalus racemosus, Pachysandra terminalis, Parthenocissus tricuspidata , Ranunculus auricomus , Rosa arkansana , and Rudbeckia sullivantii. Also new are three varieties: Crataegus irrasa var. irrasa , Crataegus pruinosa var. parvula , and Viola sagittata var. sagittata . Three species that have been reported elsewhere in 2013–2015, Isoetes viridimontana, Naias canadensis , and Solidago brendiae , are also recapitulated. This report and the recently published New Flora of Vermont (Gilman 2015) together summarize knowledge of the vascular flora of Vermont as of this date. The New Flora of Vermont was recently published by The New York Botanical Garden Press (Gilman 2015). It is the first complete accounting of the vascular flora of Vermont since 1969 (Seymour 1969) and adds more than 200 taxa to the then-known flora of the state. However, the manuscript for the New Flora was finalized in spring 2013 and additional species are now known: those that have been observed more recently, that have been recently encountered (or re-discovered) in herbaria, or that were not included because they were under study at the time of finalization. -

Journal of the Oklahoma Native Plant Society, Volume 9, December 2009
4 Oklahoma Native Plant Record Volume 9, December 2009 VASCULAR PLANTS OF SOUTHEASTERN OKLAHOMA FROM THE SANS BOIS TO THE KIAMICHI MOUNTAINS Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy May 1969 Francis Hobart Means, Jr. Midwest City, Oklahoma Current Email Address: [email protected] The author grew up in the prairie region of Kay County where he learned to appreciate proper management of the soil and the native grass flora. After graduation from college, he moved to Eastern Oklahoma State College where he took a position as Instructor in Botany and Agronomy. In the course of conducting botany field trips and working with local residents on their plant problems, the author became increasingly interested in the flora of that area and of the State of Oklahoma. This led to an extensive study of the northern portion of the Oauchita Highlands with collections currently numbering approximately 4,200. The specimens have been processed according to standard herbarium procedures. The first set has been placed in the Herbarium of Oklahoma State University with the second set going to Eastern Oklahoma State College at Wilburton. Editor’s note: The original species list included habitat characteristics and collection notes. These are omitted here but are available in the dissertation housed at the Edmon-Low Library at OSU or in digital form by request to the editor. [SS] PHYSICAL FEATURES Winding Stair Mountain ranges. A second large valley lies across the southern part of Location and Area Latimer and LeFlore counties between the The area studied is located primarily in Winding Stair and Kiamichi mountain the Ouachita Highlands of eastern ranges. -

The Native Asteraceae of Shawnee State Forest Adams and Scioto Counties, Ohio Prepared by Jim Mccormac/9/11/2018
The Native Asteraceae of Shawnee State Forest Adams and Scioto counties, Ohio Prepared by Jim McCormac/9/11/2018 1. Achillea millefolium, Yarrow 2. Ageratina altissima, White Snakeroot 3. Ageratina aromatic, Small White Snakeroot (ENDANGERED) 4. Ambrosia artemisiifolia, Common Ragweed 5. Ambrosia trifida, Common Ragweed 6. Antennaria plantaginifolia, Plantain-leaved Pussy-toes 7. Antennaria solitaria, Single-headed Pussy-toes 8. Arnoglossum atriplicifolium, Pale Indian-plantain 9. Arnoglossum muehlenbergii, Great Indian-plantain 10. Bidens bipinnata, Spanish-needles 11. Bidens cernua, Nodding Beggar’s-ticks 12. Bidens frondosa, Devil’s Beggar’s-ticks 13. Bidens polylepis, Ozark Tickseed-sunflower 14. Bidens tripartita, Purple-stemmed Beggar’s-ticks 15. Bidens vulgata, Tall Beggar’s-ticks 16. Brickellia eupatorioides, False Boneset 17. Chrysopsis mariana, Golden-aster 18. Cirsium altissimum, Tall Thistle 19. Cirsium carolinianum, Spring Thistle (THREATENED) 20. Cirsium discolor, Field Thistle 21. Conoclinium coelistinum, Mistflower 22. Conyza canadensis, Horseweed 23. Coreopsis major, Forest Tickseed 24. Coreopsis tripteris, Tall Coreopsis 25. Doellingeria umbellata, Flat-topped White Aster 26. Echinacea purpurea, Purple Coneflower 27. Eclipta prostrata, Yerba-de-tajo 28. Elephantopus carolinianus, Elephant’s-foot 29. Erechtites hieracifolia, Pilewort 30. Erigeron annuus, Daisy Fleabane 31. Erigeron philadelphicus, Philadelphia Fleabane 32. Erigeron pulchellus, Robin’s-plantain 33. Erigeron strigosus, Rough Fleabane 34. Eupatorium album, White Thoroughwort (THREATENED) 35. Eupatorium altissimum, Tall Thoroughwort 36. Eupatorium perfoliatum, Common Boneset 37. Eupatorium rotundifolium, Round-leaved Boneset 38. Eupatorium serotinum, Late-flowering Thoroughwort 39. Eupatorium sessilifolium, Upland Boneset 40. Eurybia divaricata, White Wood Aster 41. Eurybia macrophylla, Large-leaved Aster 42. Eurybia schreberi, Schreber’s Wood Aster 43. Eurybia surculosum, Creeping Aster (ENDANGERED) 44. -

Chain Bridge Flats
Maryland Native Plant Society Plant Lists We offer these lists to individuals and groups to enhance the enjoyment and study of plants of different locations in Maryland and nearby states. Their accuracy has not been verified by the Maryland Native Plant Society. CHAIN BRIDGE FLATS, WASHINGTON DC This list records plants seen during a field trip to Chain Bridge Flats near the Chain Bridge over the Potomac River on October 13, 2002. Field trip led by Cris Fleming. Plant list by Jane Hill. Nomenclature follows the USDA Plant Database at http://plants.usda.gov (January 2011). Synonyms are footnoted for some species. Agalinis purpurea Purple gerardia Scrophulariaceae Andropogon gerardii Big bluestem Poaceae Artemisia annua Annual wormwood Asteraceae Arthraxon hispidus Hairy jointgrass Poaceae Baptisia australis Blue false indigo Fabaceae Bidens aristosa1 Tickseed-sunflower Asteraceae Bidens cernua Nodding bur-marigold Asteraceae Callitriche sp. Water starwort Callitrichaceae Ceanothus herbaceus Prairie redroot Rhamnaceae Chamaecrista nictitans2 Wild sensitive plant Fabaceae Chenopodium ambrosioides Mexican tea Chenopodiaceae Cynanchum laeve Sandvine Asclepiadaceae Dichanthelium clandestinum3 Deer-tongue grass Poaceae Eleocharis compressa Flattened spike-rush Cyperaceae Elymus riparius Riverbank wild rye Poaceae Eupatorium rotundifolium4 Hairy thoroughwort Asteraceae Helianthus occidentalis Western sunflower Asteraceae Humulus japonicus Japanese hops Cannabaceae Hydrocotyle sp. Pennywort Apiaceae Hypericum prolificum 5 Shrubby St. Johnswort -

Suspected Or Known Species on Patuxent Research Refuge
Appendix A. USFWS USFWS Tree Swallow Suspected or Known Species on Patuxent Research Refuge Appendix A. Suspected or Known Species on Patuxent Research Refuge Table A-1. Suspected or Known Bird Species on Patuxent Research Refuge 1 2 Rank Rank 3 6 5 4 Heritage Heritage Status Refuge E Refuge Status & E on on T & Natural 7 Natural T 30 Common Name Scientific Name Breeding Seasons State BCR Global State Federal WATERBIRDS American Bittern Botaurus lentiginosus G4 S1 S2B I Yr M S1N Anhinga Anhinga anhinga Sp Belted Kingfisher Megaceryle alcyon Yr B Black‐crowned Night Heron Nycticorax nycticorax G5 S3B S2N SpSF M Cattle Egret Bubulcus ibis SpF Common Loon Gavia immer G5 S4N SpF Double‐crested Cormorant Phalacrocorax auritus Yr Glossy Ibis Plegadis falcinellus G5 S4B SpSF H Great Blue Heron Ardea herodias G5 S4B S3 Yr B S4N Great Egret Ardea alba G5 S4B SpSF Green Heron Butorides virescens Yr B Horned Grebe Podiceps auritus G5 S4N SpF H Least Bittern Ixobrychus exilis G5 S2 S3B I SpS B M Little Blue Heron Egretta caerulea G5 S3B SpSF M Pied‐billed Grebe Podilymbus podiceps G5 S2B S3N Yr B Red‐necked Grebe Podiceps grisegena Sp Snowy Egret Egretta thula G5 S3 S4B SpSF M White Ibis Eudocimus albus SF Yellow‐crowned Night Nyctanassa violacea G5 S2B SpF M Heron WATERFOWL American Black Duck Anas rubripes G5 S4B S5N Yr B HH American Coot Fulica americana SpFW American Wigeon Anas americana SpFW M Blue‐winged Teal Anas discors SpSF Bufflehead Bucephala albeola SpFW H Canada Goose Branta canadensis Yr ? Canvasback Aythya valisineria G5 S3 S4N SpF -
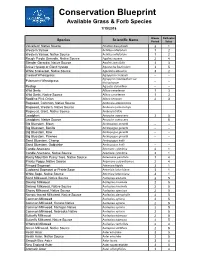
Grass and Forb Species List: 2019
Conservation Blueprint Available Grass & Forb Species 1/15/2018 Bloom Pollinator Species Scientific Name Period Value Velvetleaf, Native Source Abutilon theophrasti 2 1 Western Yarrow Achillea millefolium 1 2 Western Yarrow, Native Source Achillea millefolium 1 2 Rough Purple Gerardia, Native Source Agalinis aspera 2 4 Slender Gerardia, Native Source Agalinis tenuifolia 3 3 Anise Hyssop or Giant Hyssop Agastache foeniculum 3 5 White Snakeroot, Native Source Ageratina altissima 3 2 Crested Wheatgrass Agropyron cristatum -- -- Agropyron intermedium var. Pubescent Wheatgrass -- -- trichophorum Redtop Agrostis stolonifera -- -- Wild Garlic Allium canadense 1 3 Wild Garlic, Native Source Allium canadense 1 3 Nodding Pink Onion Allium cemuum 2 3 Ragweed, Common, Native Source Ambrosia artemisiifolia 0 0 Ragweed, Western, Native Source Ambrosia psilostachya 0 0 Ragweed, Giant, Native Source Ambrosia trifida 0 0 Leadplant Amorpha canescens 2 5 Leadplant, Native Source Amorpha canescens 0 5 Big Bluestem, Bison Andropogon gerardii -- -- Big Bluestem, Bonilla Andropogon gerardii -- -- Big Bluestem, Kaw Andropogon gerardii -- -- Big Bluestem, Pawnee Andropogon gerardii -- -- Sand Bluestem, Champ Andropogon hallii 0 0 Sand Bluestem, Goldstrike Andropogon hallii -- -- Candle Anemone Anemone cylindrica 2 1 Candle Anemone, Native Source Anemone cylindrica 2 1 Rocky Mountain Pussy Toes, Native Source Antennaria parvifolia 1 4 Prickly Poppy, Native Source Argemone polyanthemos 2 4 Fringed Sagewort Artemisia frigida 3 1 Cudweed Sagewort or Prairie Sage -

Checklist of Vascular Plants of the Southern Rocky Mountain Region
Checklist of Vascular Plants of the Southern Rocky Mountain Region (VERSION 3) NEIL SNOW Herbarium Pacificum Bernice P. Bishop Museum 1525 Bernice Street Honolulu, HI 96817 [email protected] Suggested citation: Snow, N. 2009. Checklist of Vascular Plants of the Southern Rocky Mountain Region (Version 3). 316 pp. Retrievable from the Colorado Native Plant Society (http://www.conps.org/plant_lists.html). The author retains the rights irrespective of its electronic posting. Please circulate freely. 1 Snow, N. January 2009. Checklist of Vascular Plants of the Southern Rocky Mountain Region. (Version 3). Dedication To all who work on behalf of the conservation of species and ecosystems. Abbreviated Table of Contents Fern Allies and Ferns.........................................................................................................12 Gymnopserms ....................................................................................................................19 Angiosperms ......................................................................................................................21 Amaranthaceae ............................................................................................................23 Apiaceae ......................................................................................................................31 Asteraceae....................................................................................................................38 Boraginaceae ...............................................................................................................98 -

C14 Asters.Ach-Eut
COMPOSITAE PART ONE Achillea to Eutrochium Working Draft Friday June 13th, 2015 ALPHABETICAL LIST OF PLANT MATERIALS Asteraceae Martinov formerly Compositae Adanson Asteraceae Dumortier 1822 or Compositae Giseke 1792 “As is the case, I believe, with the American Flora throughout the United States, & indeed, the whole continent, the autumnal botany of the prairies exhibits a large preponderance of the Compositae. Besides those already mentioned, we may enumerate, as of frequent occurrence, Chrysopsis mariana, Helenium autumnale, Boltonia glastifolia & B asteroides, Bidens frondosa & B chrysanthemoides, Eupatorium serotinum, E aromaticum, E ageratoides, E purpureum, &c, Cnicus glutinosus, C Virginianus, C muticus, C altissimus, &c, Silphium laciniatum, S integrifolium, S terebinthinaceum, &c, Prenanthes aspera, P virgata, P racemosa, P Uncopyrighted Draft serpentaria, &c, Vernonia fasciculata, V corymbosa, & one or two other species.” (Short 1845, Observations on the Botany of Illinois, more especially in reference to the Autumnal Flora of the Prairies.) SUNFLOWER FAMILY 1 COMPOSITAE Cichorium Achillea Cirsium Actinomeris Conoclinium Ageratina Conyza Agoseris Coreopsis Ambrosia Cosmos Anaphalis Dimorphotheca Antennaria Dracopsis Arctium Dyssodia Arnoglossum Doellingeria Artemisia Echinacea Aster Erechtites Bidens Erigeron Boltonia Eupatorium Brickellia Eurybia Cacalia Euthamia Centaurea Eutrochium Chrysanthemum Chrysopsis ASTERWORTS Every aster in my hand Goes home with a thought Emerson “A very large family of herbs, shrubs, & trees, 1500-1600 genera & 20,000-25,000 spp (formerly order Campanulales, now Asterales) considered to constitute the most highly evolved plants & characterized by florets arranged in dense heads that resemble single flowers, each floret having a gamopetalous, ligulate, or tubular corolla & a calyx modified into a pappus (as in the dandelion, sunflower, aster, & ragweed). -

Native Plants
ARLINGTON COUNTY NATIVE PLANT LIST (Version 9-1-20) The following list of vascular plants are considered native to Arlington County as initially determined through extensive flora inventories conducted from 2005-2009. This document can be considered to be accurate, but not complete. This listing will be updated periodically, identified by (Version Date), as additional species are identified. As work progresses on producing a definitive native flora list, a secondary source for checking the nativity status of a questioned species is the Digital Flora Atlas of Virginia – found at http://vaplantatlas.org/ . It is important to note that some species are periodically reclassified by the scientific community and may experience a change in genus name or family classification. Responsibility for updates and list maintenance resides with the Natural Resource Manager within the Department of Parks, Recreation and Cultural Resources (PRCR). PTERIDOPHYTA FERNS & FERN ALLIES Aspleniaceae Spleenwort Family Asplenium platyneuron Ebony Spleenwort Dennstaedtiaceae Bracken Fern Family Dennstaedtia punctilobula Hay-scented Fern Pteridium aquilinum Bracken Fern Dryopteridaceae Wood Fern Family Athyrium asplenioides Lady Fern Cystopteris protrusa Lowland Brittle Fern Deparia acrostichoides Silvery Glade Fern Dryopteris carthusiana Spinulose Wood Fern Dryopteris intermedia Evergreen Wood Fern Dryopteris marginalis Marginal Wood Fern Homalosorus pycnocarpos Matteuccia struthiopteris Ostrich Fern Onoclea sensibilis Sensitive Fern Polystichum acrostichoides Christmas