Indigo Blue Cas N°: 482-89-3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Great Tekhelet Debate—Blue Or Purple? Baruch and Judy Taubes Sterman
archaeological VIEWS The Great Tekhelet Debate—Blue or Purple? Baruch and Judy Taubes Sterman FOR ANCIENT ISRAELITES, TEKHELET WAS writings of rabbinic scholars and Greek and Roman God’s chosen color. It was the color of the sumptu- naturalists had convinced Herzog that tekhelet was a ous drapes adorning Solomon’s Temple (2 Chroni- bright sky-blue obtained from the natural secretions cles 3:14) as well as the robes worn by Israel’s high of a certain sea snail, the Murex trunculus, known to priests (Exodus 28:31). Even ordinary Israelites produce a dark purple dye.* were commanded to tie one string of tekhelet to But the esteemed chemist challenged Herzog’s the corner fringes (Hebrew, tzitzit) of their gar- contention: “I consider it impossible to produce a ments as a constant reminder of their special rela- pure blue from the purple snails that are known to Tekhelet was tionship with God (Numbers 15:38–39). me,” Friedländer said emphatically.1 But how do we know what color the Biblical writ- Unfortunately, neither Herzog nor Friedländer God’s chosen ers had in mind? While tekhelet-colored fabrics and lived to see a 1985 experiment by Otto Elsner, a color. It colored clothes were widely worn and traded throughout the chemist with the Shenkar College of Fibers in Israel, ancient Mediterranean world, by the Roman period, proving that sky-blue could, in fact, be produced the drapes donning tekhelet and similar colors was the exclusive from murex dye. During a specific stage in the dyeing of Solomon’s privilege of the emperor. -

Title: Indigo in the Modern Arab World Fulbright Hays Oman and Zanzibar
Title: Indigo in the Modern Arab World Fulbright Hays Oman and Zanzibar Program June-July 2016 Author: Victoria Vicente School: West Las Vegas Middle School, New Mexico Grade Level: 7-10 World History, United States History Purpose: This lesson is an overview of the geography of Oman and to find out more about the Burqa in Oman. There are examples provided, artwork examples, a photo analysis, an indigo dyeing activity, and readings from, “Language of Dress in the Middle East.” Activate knowledge of the region by asking students questions, for example: What is the color of the ocean? The sky? What does the color blue represent? Have you ever heard the word Burqa? Can you find the Indian Ocean on a Map? Oman? Are you wearing blue jeans? Time: 150 minutes for the lesson Extension Activity: Three days for the indigo dyeing process. Required Materials: Map of the Indian Ocean Region, Map of Oman, including Nizwa, Bahla, and Ibri, Internet, Elmo, Projector, Timeline of Indigo in Oman, show the video clips about dyeing indigo, have the students fill out the photo analysis sheets, and then for the extension activity, spend at least three days or 15 minutes per class for a week, dyeing the material. Resources: 1. Map of Oman and the Indian Ocean region http://geoalliance.asu.edu/maps/world http://cmes.arizona.edu 2. Video clips for background information: Http://www.youtube.com/watch?v=pG1zd3b7q34 http://www.youtube.com/watch?v=7z6B7ismg3k http://www.youtube.com/watch?v=Ay9g6ymhysA&feature=related 3. National Archives Photo Analysis Form or create your own. -
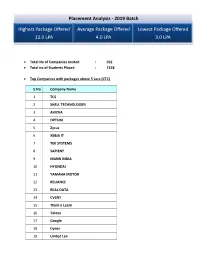
Placement Analysis - 2019 Batch
Placement Analysis - 2019 Batch Highest Package Offered Average Package Offered Lowest Package Offered 12.0 LPA 4.0 LPA 3.0 LPA Total No of Companies Invited : 502 Total no of Students Placed : 1328 Top Companies with packages above 5 Lacs (CTC) S.No Company Name 1 TCS 2 SHELL TECHNOLOGIES 3 AVIZVA 4 OPTUM 5 Zycus 6 XEBIA IT 7 TEK SYSTEMS 8 SAPIENT 9 MANN INDIA 10 HYUNDAI 11 YAMAHA MOTOR 12 RELIANCE 13 REAL DATA 14 CVENT 15 Think n Learn 16 Tolexo 17 Google 18 Dyson 19 United Lex 20 WIPRO WASE 21 HSBC 22 Accolite 23 Novanet 24 Foresight 25 Blue Star 26 RMS 27 Samsung 28 Amazon 29 Keyence 30 Veris Top Payers /Dream Companies Company Name CTC (LPA) INDIAN AIR FORCE 12 GOOGLE 12 HSBC 12 SIRION LABS 12 NATIONAL INSTRUMENTS 12 SHELL TECHNOLOGIES 10 THINK N LEARN 10 ACCOLITE 10 VMWARE 9.12 TCS (CODEVITA) 7.5 HYUNDAI 7.5 NOVANET 7 ZYCUS 6.5 XEBIA IT 6 MANN INDIA TECHNOLOGIES 6 RELIANCE 6 REAL DATA 6 FORESIGHT 6 BLUE STAR 6 SAMSUNG 6 AMAZON 6 KEYENCE 6 WIPRO WASE 5.57 OPTUM 5.4 SAPIENT 5.3 AVIZVA 5.25 DYSON 5.07 YAMAHA 5 CVENT 5 TOLEXO 5 UNITED LEX 5 PFIZER 5 LAMBORGINI 5 Top Recruiters Company Name Final Placed ACCENTURE 309 TCS 120 WIPRO TECHNOLOGIES 70 THINK N LEARN 40 ICONMA 39 NEC TECHNOLOGIES 29 AMAZON 52 FLIPKART 25 RELIANCE 23 MANN INDIA TECHNOLOGIES 21 SOPRA STERIA 20 HEXAWARE 16 HCL TECHNOLOGIES 23 SAMSUNG 28 ERICSSON 15 NTT DATA 33 Top IT Companies: S.No Company Name 1 NEC 2 TATA TECHNOLOGIES 3 WIPRO 4 ACCENTURE 5 HEXAWARE 6 OPTUM 7 NIIT 8 GRAPECITY 9 TCS 10 MPHASIS 11 SAPIENT 12 NEWGEN 13 ACCENTURE 14 Gemalto 15 Google 16 Genpact -

Flags and Symbols � � � Gilbert Baker Designed the Rainbow flag for the 1978 San Francisco’S Gay Freedom Celebration
Flags and Symbols ! ! ! Gilbert Baker designed the rainbow flag for the 1978 San Francisco’s Gay Freedom Celebration. In the original eight-color version, pink stood for sexuality, red for life, orange for healing, yellow for the sun, green for nature, turquoise for art, indigo for harmony and violet for the soul.! " Rainbow Flag First unveiled on 12/5/98 the bisexual pride flag was designed by Michael Page. This rectangular flag consists of a broad magenta stripe at the top (representing same-gender attraction,) a broad stripe in blue at the bottoms (representing opposite- gender attractions), and a narrower deep lavender " band occupying the central fifth (which represents Bisexual Flag attraction toward both genders). The pansexual pride flag holds the colors pink, yellow and blue. The pink band symbolizes women, the blue men, and the yellow those of a non-binary gender, such as a gender bigender or gender fluid Pansexual Flag In August, 2010, after a process of getting the word out beyond the Asexual Visibility and Education Network (AVEN) and to non-English speaking areas, a flag was chosen following a vote. The black stripe represents asexuality, the grey stripe the grey-are between sexual and asexual, the white " stripe sexuality, and the purple stripe community. Asexual Flag The Transgender Pride flag was designed by Monica Helms. It was first shown at a pride parade in Phoenix, Arizona, USA in 2000. The flag represents the transgender community and consists of five horizontal stripes. Two light blue which is the traditional color for baby boys, two pink " for girls, with a white stripe in the center for those Transgender Flag who are transitioning, who feel they have a neutral gender or no gender, and those who are intersex. -

Auto Yearbook FY20
AutoAuto Yearbook FY20 April 30, 2020 Section I: Update – What went down? FY20 was a tough year for the entire Indian automotive industry. Myriad demand and supply side issues continued to trouble the space, continuing the system-wide weakness that set in around the 2018 festive period. Broad- based decline in OEM volumes (Exhibit 1) throughout the year encapsulated the pain at that level as well as the knock-on impact on supporting Report ecosystems of ancillaries, on the one hand, and dealerships, on the other. Higher cost incidence and general reluctance in consumer spending affected the PV and 2-W segments most, while slowing economic activity and system pecial overcapacity took a toll on CV segment. OEM focus on inventory destocking S in the run up to BS-VI switchover from April 2020 and Covid-19 outbreak were other issues that adversely impacted Q4FY20, in particular. Total industry volumes fell 14.8% YoY to 2.63 crore – one of the worst performances in decades, with all major segments registering hefty declines- PV - Down 14.8% YoY to 34.53 lakh units, was dragged by 19.9% dip in passenger cars and 39% decline in vans. UV sub segment, however, posted 2.7% growth courtesy several successful new Research Analysts product launches CV – Was hardest hit, down 29.7% YoY to 7.78 lakh units. M&HCV Shashank Kanodia, CFA [email protected] sub segment dropped 43.3% amid persistent weakness in trucks (down 49.1%) while buses bucked the trend (up 3.5%). LCV sub Jaimin Desai [email protected] segment came off by 20.7% with both – passenger and goods categories in the red 3-W – Was down 10.2% YoY to 11.39 lakh units amid double digit declines in passenger as well as goods categories 2-W – Was down 14.4% YoY to 2.1 crore units. -

Indigo Planting & Harvesting
Indigo Planting & Harvesting Nicholas Wenner November 2017 Photo by Paige Green by Paige Photo Table of Contents Introduction . .3 Planting . .4 Growing Seedlings . .4 Broadcasting Seedlings . .4 Planting in Flats . .4 Sources for Seeds . 5 Transplanting . .5 Transplanting by Hand . .6 Hand-Held Tools . .6 Walk-Behind Machines . 7 Semi-Automated Tractor-Mounted Machines . .9 Fully-Automated Machines . 11 Harvesting . 12 Harvesting by Hand . .13 Walk-Behind Machines . .14 Tractor Mounted and Self-Propelled Machines . 17 Summary and Recommendations . 20 Planting . 20 Harvesting . .21 Summary and Recommendations . .21 Indigo: Planting & Harvesting 2 Introduction his report was completed with funding generously provided by the Jena and Michael TKing Foundation as part of Fibershed’s True Blue project . The project aims to support bio-regional manufacturing systems for natural indigo dye and indigo-dyed textiles in the Northern California fibershed and beyond . It is one project of many that support Fibershed’s larger mission: “Fibershed develops regional and regenerative fiber systems on behalf of independent working producers, by expanding opportunities to implement carbon farming, forming catalytic foundations to rebuild regional manufacturing, and through connecting end-users to farms and ranches through public education.” In particular, this document aims to support regional farmers in their efforts to bring indigo farming forward as a viable economic option . We give a detailed review of the planting and harvesting steps of indigo dye production and consider how to scale production using appropriate tools and technology . We focus on planting and harvesting because these steps have represented significant bottlenecks in indigo production in Northern California due to the labor content of the manual methods currently employed . -

Indigo Soars with $33Bn A320neo Order
EEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEE THE HINDU DELHI WEDNESDAY, OCTOBER 30, 2019 BUSINESS 15 EEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEE market watch Awaiting clarity on AGR, 29-10-2019 % CHANGE Sensex dddddddddddddddddddddd 39,832 ddddddddddddddd1.48 IndiGo soars with $33bn A320neo order US Dollardddddddddddddddddddd 70.84 ddddddddddddddd0.08 Gold ddddddddddddddddddddddddddd 38,857 ddddddddddddd-1.39 Bharti defers Q2 results Brent oil ddddddddddddddddddddd 61.62 ddddddddddddddd0.16 300plane order is Airbus’ largest ever with a single airline; to take IndiGo’s A320neo count to 730 ‘Moving DoT to arrive at total amount’ SPECIAL CORRESPONDENT economic growth and mobil IndiGo is keen on the MUMBAI ity,” Ronojoy Dutta, CEO, In A321XLR, which is the next Special Correspondent matter arising out of recent N IFTY 50 InterGlobe Aviation Ltd., diGo, said in a statement. evolutionary -

Indigo Promoter Feud out in the Open
EEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEE THE HINDU DELHI WEDNESDAY, JULY 10, 2019 BUSINESS 15 EEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEE market watch 09-07-2019 % CHANGE Sensex dddddddddddddddddddddd 38,731 ddddddddddddddd0.03 IndiGo promoter feud out in the open US Dollardddddddddddddddddddd 68.51 ddddddddddddddd0.21 Gold ddddddddddddddddddddddddddd 34,870 ddddddddddddd-1.69 Brent oil ddddddddddddddddddddd 64.19 ddddddddddddd-0.81 Rakesh Gangwal writes to markets regulator SEBI, flags ‘unusual rights’ available to Rahul Bhatia SPECIAL CORRESPONDENT I request SEBI to which IGE has a share. NEW DELHI <> ask the firm to “I hadn't contemplated The war between IndiGo’s make changes to over that over the years, Mr. two promoters Rahul Bhatia Bhatia would start building NIFTY 50 the unusual rights and Rakesh Gangwal is now an ecosystem of other com PRICE CHANGE that IGE Group has Adani -

On Indigo in the Urine
Article V.? -On Indigo in the Urine. By W. Gilchrist, M.D., T orquay. There are several cases on record in which a blue deposit lias been observed in the urine, but these are so few in number that I believe the following notes of such a case may not be uninteresting. The patient is a poor woman residing at Chris tow, a small village in Devonshire. For some years she has been under the care of my friend Dr Lillies of Chudleigh, through whose kindness I have been enabled to see the patient, to obtain specimens of the urine for examination, and to give the following notes of her case :? I shall first describe the urine. The urine is of a dirty greenish yellow colour, strongly ammonia- cal; Sp. grav. 1010. On standing some time it deposits a dirty greenish blue sediment, which to the naked eye seems to be com- posed?1 st, Of a heavy white crystalline deposit; 2d, Of whitish flakes of organic matter; and 3d, Adhering to or mixed with the latter, particles or patches of a deep blue colour. Microscopic in- ? spection proves the white deposit to consist of the triple phosphates the flakes of organic matter to consist of tessellated epithelial cells, which are swollen, granular and opaque, so that the nucleus is in- " visible. These cells are in the state which Virchow terms cloudy swelling." The blue matter under the microscope appears amorphous, and presents two distinct shades?a light and a dark blue?both in brightness of tint resembling rather the shades of Prussian blue than those of indigo. -

Indigo Bunting Passerina Cyanea
indigo bunting Passerina cyanea Kingdom: Animalia FEATURES Phylum: Chordata The indigo bunting averages five and one-half inches Class: Aves in length (tail tip to bill tip in preserved specimen). Order: Passeriformes The female has brown feathers. The male has brilliant blue feathers in breeding season and a Family: Cardinalidae mixture of brown and blue feathers the rest of the ILLINOIS STATUS year. common, native BEHAVIORS The indigo bunting is a common migrant and summer resident statewide and a rare winter resident in the southern one-third of Illinois. A bird of open areas, it can be found in cultivated fields but is more common near bottomland forests. Its song can be heard throughout the day in the spring and summer. The song includes different pitches usually with paired notes, like “sweet-sweet” or “chew- chew.” The indigo bunting migrates at night, with spring migrants beginning to arrive in April. The males arrive before the females. The breeding season occurs from May through mid-August. The nest is built by the female in dense vegetation close to the ground and composed of dried grasses, bark strips, twigs and other plant materials. It is lined with grasses, rootlets, hair and feathers. The female may use a snake skin in the nest base. Three or four, pale-blue eggs are deposited by the female, and she alone incubates them over the 12- to 13-day incubation period. Two broods may be raised in one year. The nest is often parasitized by the brown- headed cowbird that deposits an egg that the indigo bunting will hatch and raise, taking food and care away from its own young. -

Sharekhan Special August 31, 2021
Sharekhan Special August 31, 2021 Index Q1FY2022 Results Review Automobiles • Capital Goods • Consumer Discretionary • Consumer Goods • Infrastructure/Cement/Logistics/Building Material • IT • Oil & Gas • Pharmaceuticals • Agri Inputs and Speciality Chemical • Miscellaneous • Visit us at www.sharekhan.com For Private Circulation only Q1FY2022 Results Review In-line quarter, healthy outlook Results Review Results Summary: After ending FY2021 on a strong note, Q1FY2022 earnings of broader indices showed a promising start (Nifty/ Sensex companies’ PAT rose 100%/66% y-o-y) in the new fiscal with strong growth momentum on low base. Management commentaries on earnings outlook remained positive, on improving economic activity post second COVID-19 wave and anticipation of strong demand revival. Demand recovery and ramp-up of vaccinations look encouraging. We expect economic activity to increase in the upcoming festive season. Nifty trades at 23x and 20x EPS based on FY2022E/FY2023E EPS, at a premium to mean average. Valuation gap between large and mid-caps has shrunk, we advise investors to focus on stocks with strong earnings growth potential with reasonable valuation. High-conviction investment ideas: o Large-caps: Infosys, ICICI Bank, M&M, L&T, UltraTech, SBI, HDFC Ltd, Godrej Consumer Products, Divis Labs and Titan. o Mid-caps: NAM India, BEL, Gland Pharma, Dalmia Bharat, Laurus Labs, Max Financial Services, LTI. o Small-caps: TCI Express, Kirloskar Oil, Suprajit Engineering, Repco Home Finance, PNC Infratech, Mahindra Lifespaces, Birlasoft. After ending FY2021 on a strong note, Q1FY2022 corporate earnings of broader indices showed a promising start with continued strong growth momentum on the low base of Q1FY2021, though it was along the expected lines. -
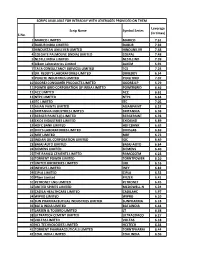
S.No. Scrip Name Symbol Series Leverage (In Times) 1 MARICO
SCRIPS AVAILABLE FOR INTRADAY WITH LEVERAGES PROVIDED ON THEM Leverage Scrip Name Symbol Series (in times) S.No. 1 MARICO LIMITED MARICO 7.61 2 DABUR INDIA LIMITED DABUR 7.92 3 HINDUSTAN UNILEVER LIMITED HINDUNILVR 7.48 4 COLGATE PALMOLIVE (INDIA) LIMITED COLPAL 7.48 5 NESTLE INDIA LIMITED NESTLEIND 7.39 6 Alkem Laboratories Limited ALKEM 6.91 7 TATA CONSULTANCY SERVICES LIMITED TCS 7.24 8 DR. REDDY'S LABORATORIES LIMITED DRREDDY 6.54 9 PIDILITE INDUSTRIES LIMITED PIDILITIND 7.07 10 GODREJ CONSUMER PRODUCTS LIMITED GODREJCP 5.79 11 POWER GRID CORPORATION OF INDIA LIMITED POWERGRID 6.46 12 ACC LIMITED ACC 6.61 13 NTPC LIMITED NTPC 6.64 14 ITC LIMITED ITC 7.05 15 ASIAN PAINTS LIMITED ASIANPAINT 6.52 16 BRITANNIA INDUSTRIES LIMITED BRITANNIA 6.98 17 BERGER PAINTS (I) LIMITED BERGEPAINT 6.78 18 EXIDE INDUSTRIES LIMITED EXIDEIND 6.89 19 HDFC BANK LIMITED HDFCBANK 6.63 20 DIVI'S LABORATORIES LIMITED DIVISLAB 6.69 21 MRF LIMITED MRF 6.73 22 INDIAN OIL CORPORATION LIMITED IOC 6.49 23 BAJAJ AUTO LIMITED BAJAJ-AUTO 6.64 24 SIEMENS LIMITED SIEMENS 6.40 25 THE RAMCO CEMENTS LIMITED RAMCOCEM 6.23 26 TORRENT POWER LIMITED TORNTPOWER 6.10 27 UNITED BREWERIES LIMITED UBL 6.16 28 INFOSYS LIMITED INFY 6.82 29 CIPLA LIMITED CIPLA 6.52 30 Pfizer Limited PFIZER 6.41 31 PETRONET LNG LIMITED PETRONET 6.45 32 UNITED SPIRITS LIMITED MCDOWELL-N 6.24 33 CADILA HEALTHCARE LIMITED CADILAHC 5.97 34 WIPRO LIMITED WIPRO 6.10 35 SUN PHARMACEUTICAL INDUSTRIES LIMITED SUNPHARMA 6.18 36 BATA INDIA LIMITED BATAINDIA 6.44 37 LARSEN & TOUBRO LIMITED LT 6.38 38 ULTRATECH CEMENT