Sedar41-Rd41
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

Fish Populations and Habitat Assessment on the Oculina Bank
195 Abstract—A portion of the Oculina Assessment of fish populations and habitat Bank located off eastern Florida is a marine protected area (MPA) pre- on Oculina Bank, a deep-sea coral marine served for its dense populations of the ivory tree coral (Oculina varicosa), protected area off eastern Florida which provides important habitat for fish. Surveys of fish assemblages Stacey L. Harter (contact author)1 and benthic habitat were conducted 1 inside and outside the MPA in 2003 Marta M. Ribera and 2005 by using remotely operated Andrew N. Shepard2 vehicle video transects and digital 3 still imagery. Fish species composi- John K. Reed tion, biodiversity, and grouper densi- Email address for contact author: [email protected] ties were used to determine whether 1 National Marine Fisheries Service O. varicosa forms an essential habitat Southeast Fisheries Science Center compared to other structure-forming 3500 Delwood Beach Rd. habitats and to examine the effective- Panama City, Florida 32408 ness of the MPA. Multivariate analy- 2 NOAA Undersea Research Center ses indicated no differences in fish University of North Carolina at Wilmington assemblages or biodiversity among 5600 Marvin Moss Lane hardbottom habitat types and grou- Wilmington, North Carolina 28409 per densities were highest among the most complex habitats; however the 3 Harbor Branch Oceanographic Institute higher densities were not exclusive to Florida Atlantic University coral habitat. Therefore, we conclude 5600 U.S. 1 North that O. varicosa was functionally Ft. Pierce, Florida 34946 equivalent to other hardbottom habi- tats. Even though fish assemblages were not different among manage- ment areas, biodiversity and grouper densities were higher inside the MPA compared to outside. -

Ices/Nafo Wgdec Report 2015
ICES/NAFO WGDEC REPORT 2015 ICES ADVISORY COMMITTEE ICES CM 2015/ACOM:27 Report of the ICES/NAFO Joint Working Group on Deep-water Ecology (WGDEC) 16–20 February 2015 Horta, Azores, Portugal International Council for the Exploration of the Sea Conseil International pour l’Exploration de la Mer H. C. Andersens Boulevard 44–46 DK-1553 Copenhagen V Denmark Telephone (+45) 33 38 67 00 Telefax (+45) 33 93 42 15 www.ices.dk [email protected] Recommended format for purposes of citation: ICES. 2015. Report of the ICES/NAFO Joint Working Group on Deep-water Ecology (WGDEC), 16–20 February 2015, Horta, Azores, Portugal. ICES CM 2015/ACOM:27. 113 pp. For permission to reproduce material from this publication, please apply to the Gen- eral Secretary. The document is a report of an Expert Group under the auspices of the International Council for the Exploration of the Sea and does not necessarily represent the views of the Council. © 2015 International Council for the Exploration of the Sea ICES/NAFO WGDEC REPORT 2015 | i Contents Executive summary ................................................................................................................ 4 Opening of the meeting ........................................................................................................ 5 1 Adoption of the agenda ................................................................................................ 6 2 Provide all available new information on distribution of VMEs in the North Atlantic with a view to advising on any new closures to bottom fisheries or revision of existing closures to bottom fisheries (NEAFC standing request). In addition, provide new information on location of habitats sensitive to particular fishing activities (i.e. vulnerable marine ecosystems, VMEs) within EU waters (EC request) ................................... 8 2.1 Introduction ........................................................................................................... 8 2.2 Areas within the NEAFC regulatory area ........................................................ -

Fish Assemblages Associated with Red Grouper Pits at Pulley Ridge, A
419 Abstract—Red grouper (Epineph- elus morio) modify their habitat by Fish assemblages associated with red grouper excavating sediment to expose rocky pits, providing structurally complex pits at Pulley Ridge, a mesophotic reef in the habitat for many fish species. Sur- Gulf of Mexico veys conducted with remotely op- erated vehicles from 2012 through 2015 were used to characterize fish Stacey L. Harter (contact author)1 assemblages associated with grouper Heather Moe1 pits at Pulley Ridge, a mesophotic 2 coral ecosystem and habitat area John K. Reed of particular concern in the Gulf Andrew W. David1 of Mexico, and to examine whether invasive species of lionfish (Pterois Email address for contact author: [email protected] spp.) have had an effect on these as- semblages. Overall, 208 grouper pits 1 Southeast Fisheries Science Center were examined, and 66 fish species National Marine Fisheries Service, NOAA were associated with them. Fish as- 3500 Delwood Beach Road semblages were compared by using Panama City, Florida 32408 several factors but were considered 2 Harbor Branch Oceanographic Institute to be significantly different only on Florida Atlantic University the basis of the presence or absence 5600 U.S. 1 North of predator species in their pit (no Fort Pierce, Florida 34946 predators, lionfish only, red grou- per only, or both lionfish and red grouper). The data do not indicate a negative effect from lionfish. Abun- dances of most species were higher in grouper pits that had lionfish, and species diversity was higher in grouper pits with a predator (lion- The red grouper (Epinephelus morio) waters (>70 m) of the shelf edge and fish, red grouper, or both). -

Drum and Croaker (Family Sciaenidae) Diversity in North Carolina
Drum and Croaker (Family Sciaenidae) Diversity in North Carolina The waters along and off the coast are where you will find 18 of the 19 species within the Family Sciaenidae (Table 1) known from North Carolina. Until recently, the 19th species and the only truly freshwater species in this family, Freshwater Drum, was found approximately 420 miles WNW from Cape Hatteras in the French Broad River near Hot Springs. Table 1. Species of drums and croakers found in or along the coast of North Carolina. Scientific Name/ Scientific Name/ American Fisheries Society Accepted Common Name American Fisheries Society Accepted Common Name Aplodinotus grunniens – Freshwater Drum Menticirrhus saxatilis – Northern Kingfish Bairdiella chrysoura – Silver Perch Micropogonias undulatus – Atlantic Croaker Cynoscion nebulosus – Spotted Seatrout Pareques acuminatus – High-hat Cynoscion nothus – Silver Seatrout Pareques iwamotoi – Blackbar Drum Cynoscion regalis – Weakfish Pareques umbrosus – Cubbyu Equetus lanceolatus – Jackknife-fish Pogonias cromis – Black Drum Larimus fasciatus – Banded Drum Sciaenops ocellatus – Red Drum Leiostomus xanthurus – Spot Stellifer lanceolatus – Star Drum Menticirrhus americanus – Southern Kingfish Umbrina coroides – Sand Drum Menticirrhus littoralis – Gulf Kingfish With so many species historically so well-known to recreational and commercial fishermen, to lay people, and their availability in seafood markets, it is not surprising that these 19 species are known by many local and vernacular names. Skimming through the ETYFish Project -

Phylogeny of the Epinephelinae (Teleostei: Serranidae)
BULLETIN OF MARINE SCIENCE, 52(1): 240-283, 1993 PHYLOGENY OF THE EPINEPHELINAE (TELEOSTEI: SERRANIDAE) Carole C. Baldwin and G. David Johnson ABSTRACT Relationships among epinepheline genera are investigated based on cladistic analysis of larval and adult morphology. Five monophyletic tribes are delineated, and relationships among tribes and among genera of the tribe Grammistini are hypothesized. Generic com- position of tribes differs from Johnson's (1983) classification only in the allocation of Je- boehlkia to the tribe Grammistini rather than the Liopropomini. Despite the presence of the skin toxin grammistin in the Diploprionini and Grammistini, we consider the latter to be the sister group of the Liopropomini. This hypothesis is based, in part, on previously un- recognized larval features. Larval morphology also provides evidence of monophyly of the subfamily Epinephelinae, the clade comprising all epinepheline tribes except Niphonini, and the tribe Grammistini. Larval features provide the only evidence of a monophyletic Epine- phelini and a monophyletic clade comprising the Diploprionini, Liopropomini and Gram- mistini; identification of larvae of more epinephelines is needed to test those hypotheses. Within the tribe Grammistini, we propose that Jeboehlkia gladifer is the sister group of a natural assemblage comprising the former pseudogrammid genera (Aporops, Pseudogramma and Suttonia). The "soapfishes" (Grammistes, Grammistops, Pogonoperca and Rypticus) are not monophyletic, but form a series of sequential sister groups to Jeboehlkia, Aporops, Pseu- dogramma and Suttonia (the closest of these being Grammistops, followed by Rypticus, then Grammistes plus Pogonoperca). The absence in adult Jeboehlkia of several derived features shared by Grammistops, Aporops, Pseudogramma and Suttonia is incongruous with our hypothesis but may be attributable to paedomorphosis. -

Zootaxa, Marine Fish Diversity: History of Knowledge and Discovery
Zootaxa 2525: 19–50 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) Marine fish diversity: history of knowledge and discovery (Pisces) WILLIAM N. ESCHMEYER1, 5, RONALD FRICKE2, JON D. FONG3 & DENNIS A. POLACK4 1Curator emeritus, California Academy of Sciences, San Francisco, California, U.S.A. 94118 and Research Associate, Florida Museum of Natural History, Gainesville, Florida, U.S.A. 32611. E-mail: [email protected] 2Ichthyology, Staatliches Museum für Naturkunde, Rosenstein 1, 70191 Stuttgart, Germany. E-mail: [email protected] 3California Academy of Sciences, San Francisco, California, U.S.A. 94118. E-mail: [email protected] 4P.O. Box 518, Halfway House 1685, South Africa. E-mail: [email protected] 5Corresponding Author. E-mail: [email protected] Abstract The increase in knowledge of marine fish biodiversity over the last 250 years is assessed. The Catalog of Fishes database (http://research.calacademy.org/ichthyology/catalog) on which this study is based, has been maintained for 25 years and includes information on more than 50,000 available species names of fishes, with more than 31,000 of them currently regarded as valid species. New marine species are being described at a rate of about 100–150 per year, with freshwater numbers slightly higher. In addition, over 10,000 generic names are available ones of which 3,118 are deemed valid for marine fishes (as of Feb. 19, 2010). This report concentrates on fishes with at least some stage of their life cycle in the sea. The number of valid marine species, about 16,764 (Feb. -

Venom Evolution Widespread in Fishes: a Phylogenetic Road Map for the Bioprospecting of Piscine Venoms
Journal of Heredity 2006:97(3):206–217 ª The American Genetic Association. 2006. All rights reserved. doi:10.1093/jhered/esj034 For permissions, please email: [email protected]. Advance Access publication June 1, 2006 Venom Evolution Widespread in Fishes: A Phylogenetic Road Map for the Bioprospecting of Piscine Venoms WILLIAM LEO SMITH AND WARD C. WHEELER From the Department of Ecology, Evolution, and Environmental Biology, Columbia University, 1200 Amsterdam Avenue, New York, NY 10027 (Leo Smith); Division of Vertebrate Zoology (Ichthyology), American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024-5192 (Leo Smith); and Division of Invertebrate Zoology, American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024-5192 (Wheeler). Address correspondence to W. L. Smith at the address above, or e-mail: [email protected]. Abstract Knowledge of evolutionary relationships or phylogeny allows for effective predictions about the unstudied characteristics of species. These include the presence and biological activity of an organism’s venoms. To date, most venom bioprospecting has focused on snakes, resulting in six stroke and cancer treatment drugs that are nearing U.S. Food and Drug Administration review. Fishes, however, with thousands of venoms, represent an untapped resource of natural products. The first step in- volved in the efficient bioprospecting of these compounds is a phylogeny of venomous fishes. Here, we show the results of such an analysis and provide the first explicit suborder-level phylogeny for spiny-rayed fishes. The results, based on ;1.1 million aligned base pairs, suggest that, in contrast to previous estimates of 200 venomous fishes, .1,200 fishes in 12 clades should be presumed venomous. -

Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes
Old Dominion University ODU Digital Commons Biological Sciences Theses & Dissertations Biological Sciences Summer 2016 Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes Christi Linardich Old Dominion University, [email protected] Follow this and additional works at: https://digitalcommons.odu.edu/biology_etds Part of the Biodiversity Commons, Biology Commons, Environmental Health and Protection Commons, and the Marine Biology Commons Recommended Citation Linardich, Christi. "Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes" (2016). Master of Science (MS), Thesis, Biological Sciences, Old Dominion University, DOI: 10.25777/hydh-jp82 https://digitalcommons.odu.edu/biology_etds/13 This Thesis is brought to you for free and open access by the Biological Sciences at ODU Digital Commons. It has been accepted for inclusion in Biological Sciences Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. HOTSPOTS, EXTINCTION RISK AND CONSERVATION PRIORITIES OF GREATER CARIBBEAN AND GULF OF MEXICO MARINE BONY SHOREFISHES by Christi Linardich B.A. December 2006, Florida Gulf Coast University A Thesis Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE BIOLOGY OLD DOMINION UNIVERSITY August 2016 Approved by: Kent E. Carpenter (Advisor) Beth Polidoro (Member) Holly Gaff (Member) ABSTRACT HOTSPOTS, EXTINCTION RISK AND CONSERVATION PRIORITIES OF GREATER CARIBBEAN AND GULF OF MEXICO MARINE BONY SHOREFISHES Christi Linardich Old Dominion University, 2016 Advisor: Dr. Kent E. Carpenter Understanding the status of species is important for allocation of resources to redress biodiversity loss. -

Field Guide to the Searobins in the Western North Atlantic
NOAA Technical Report NMFS 107 March 1992 Field Guide to the Searobins (Prionotus and Bellator) in the Western North Atlantic Mike Russell Mark Grace ElmerJ. Gutherz U.S. Department ofCommerce NOAA Technical Report NMFS The major responsibilities ofthe National Marine Fish continuing programs ofNMFS; intensive scientific reports eries Service (NMFS) are to monitor and assess the abun on studies of restricted scope; papers on applied fishery dance and geographic distribution of fishery resources, problems; technical reports of general interest intended to understand and predict fluctuations in the quantity to aid conservation and management; reports that re and distribution ofthese resources, and to establish levels view, in considerable detail and at high technical level, for their optimum use. NMFS is also charged with the certain broad areas ofresearch; and technical papers origi development and implementation of policies for manag nating in economic studies and in management investi ing national fishing grounds, with the development and gations. Since this is a formal series, all submitted papers, enforcement of domestic fisheries regulations, with the except those of the U.S.-Japan series on aquaculture, surveillance offoreign fishing off U.S. coastal waters, and receive peer review and all papers, once accepted, re with the development and enforcement of international ceive professional editing before publication. fishery agreements and policies. NMFS also assists the fishing industry through marketing service and economic Copies of NOAA Technical Reports NMFS are avail analysis programs and through mortgage insurance and able free in limited numbers to government agencies, vessel construction subsidies. Itcollects, analyzes, and pub both federal and state. -

FISHES (C) Val Kells–November, 2019
VAL KELLS Marine Science Illustration 4257 Ballards Mill Road - Free Union - VA - 22940 www.valkellsillustration.com [email protected] STOCK ILLUSTRATION LIST FRESHWATER and SALTWATER FISHES (c) Val Kells–November, 2019 Eastern Atlantic and Gulf of Mexico: brackish and saltwater fishes Subject to change. New illustrations added weekly. Atlantic hagfish, Myxine glutinosa Sea lamprey, Petromyzon marinus Deepwater chimaera, Hydrolagus affinis Atlantic spearnose chimaera, Rhinochimaera atlantica Nurse shark, Ginglymostoma cirratum Whale shark, Rhincodon typus Sand tiger, Carcharias taurus Ragged-tooth shark, Odontaspis ferox Crocodile Shark, Pseudocarcharias kamoharai Thresher shark, Alopias vulpinus Bigeye thresher, Alopias superciliosus Basking shark, Cetorhinus maximus White shark, Carcharodon carcharias Shortfin mako, Isurus oxyrinchus Longfin mako, Isurus paucus Porbeagle, Lamna nasus Freckled Shark, Scyliorhinus haeckelii Marbled catshark, Galeus arae Chain dogfish, Scyliorhinus retifer Smooth dogfish, Mustelus canis Smalleye Smoothhound, Mustelus higmani Dwarf Smoothhound, Mustelus minicanis Florida smoothhound, Mustelus norrisi Gulf Smoothhound, Mustelus sinusmexicanus Blacknose shark, Carcharhinus acronotus Bignose shark, Carcharhinus altimus Narrowtooth Shark, Carcharhinus brachyurus Spinner shark, Carcharhinus brevipinna Silky shark, Carcharhinus faiformis Finetooth shark, Carcharhinus isodon Galapagos Shark, Carcharhinus galapagensis Bull shark, Carcharinus leucus Blacktip shark, Carcharhinus limbatus Oceanic whitetip shark, -
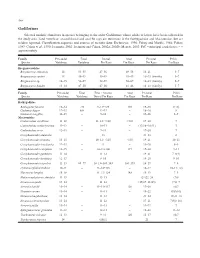
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri