Northern Riffleshell
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tennessee's Extinct Species
Tennessee's Extinct Species The following species Birds: once occurred in Carolina parakeet Conuropsis carolinensis Ectopistes migratorius Tennessee and are now Passenger pigeon believed to be extinct. Mammals: Following this list are two Eastern elk species descriptions-one Fishes: describing the Carolina Harelip sucker parakeet and another describing the extinct Mussels: Acornshell Epioblasma haysiana freshwater mussels Angled riffleshell Epioblasma biemarginata of Tennessee. Cumberland leafshell Epioblasma stewardsoni Leafshell Epioblasma flexuosa Narrowcat's paw Epioblasma lenoir Rough rockshell Quadrula tuberosa Round combshell Epioblasma personata Sugarspoon Epioblasma arcaeformis Tennessee riffleshell Epioblasma propinqua Carolina Parakeet Status Habitat The Carolina parakeet is an The Carolina parakeet was found Learn rrwreabout extinct species. in riverine forests, cypress swamps, Tennessee's diverse and other woodlands over much of Description the Eastern and Midwest Regions of ecosyster.n3.Su~ort The Carolina parakeet was a the United States. It was the only conservation in your small parrot, about 12inches in parrot native to the United States. community and state! length. Its head was lemon yellow, The parakeets rested at night in with an orange forehead and cheeks. groups, with as many as 30 birds The rest of its body was green. Its sleeping inside one hollowtree, while legs and beak were pale pinkish- others would hang on the outside. white. These curious birds lived and Nests were placed in hollowtrees, traveled in flocks. and three to five white eggs were laid. Up to 50 nests were often crowded into one tree. Role in the Ecosystem Carolina parakeets enjoyed a variety of different foods-apples, peaches, mulberries, pecans, grapes, dogwood fruit, and grains. -

ECOLOGY of NORTH AMERICAN FRESHWATER FISHES
ECOLOGY of NORTH AMERICAN FRESHWATER FISHES Tables STEPHEN T. ROSS University of California Press Berkeley Los Angeles London © 2013 by The Regents of the University of California ISBN 978-0-520-24945-5 uucp-ross-book-color.indbcp-ross-book-color.indb 1 44/5/13/5/13 88:34:34 AAMM uucp-ross-book-color.indbcp-ross-book-color.indb 2 44/5/13/5/13 88:34:34 AAMM TABLE 1.1 Families Composing 95% of North American Freshwater Fish Species Ranked by the Number of Native Species Number Cumulative Family of species percent Cyprinidae 297 28 Percidae 186 45 Catostomidae 71 51 Poeciliidae 69 58 Ictaluridae 46 62 Goodeidae 45 66 Atherinopsidae 39 70 Salmonidae 38 74 Cyprinodontidae 35 77 Fundulidae 34 80 Centrarchidae 31 83 Cottidae 30 86 Petromyzontidae 21 88 Cichlidae 16 89 Clupeidae 10 90 Eleotridae 10 91 Acipenseridae 8 92 Osmeridae 6 92 Elassomatidae 6 93 Gobiidae 6 93 Amblyopsidae 6 94 Pimelodidae 6 94 Gasterosteidae 5 95 source: Compiled primarily from Mayden (1992), Nelson et al. (2004), and Miller and Norris (2005). uucp-ross-book-color.indbcp-ross-book-color.indb 3 44/5/13/5/13 88:34:34 AAMM TABLE 3.1 Biogeographic Relationships of Species from a Sample of Fishes from the Ouachita River, Arkansas, at the Confl uence with the Little Missouri River (Ross, pers. observ.) Origin/ Pre- Pleistocene Taxa distribution Source Highland Stoneroller, Campostoma spadiceum 2 Mayden 1987a; Blum et al. 2008; Cashner et al. 2010 Blacktail Shiner, Cyprinella venusta 3 Mayden 1987a Steelcolor Shiner, Cyprinella whipplei 1 Mayden 1987a Redfi n Shiner, Lythrurus umbratilis 4 Mayden 1987a Bigeye Shiner, Notropis boops 1 Wiley and Mayden 1985; Mayden 1987a Bullhead Minnow, Pimephales vigilax 4 Mayden 1987a Mountain Madtom, Noturus eleutherus 2a Mayden 1985, 1987a Creole Darter, Etheostoma collettei 2a Mayden 1985 Orangebelly Darter, Etheostoma radiosum 2a Page 1983; Mayden 1985, 1987a Speckled Darter, Etheostoma stigmaeum 3 Page 1983; Simon 1997 Redspot Darter, Etheostoma artesiae 3 Mayden 1985; Piller et al. -
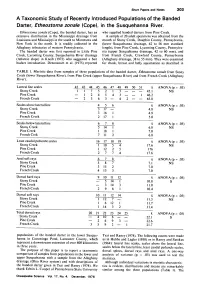
A Taxonomic Study of Recently Introduced Populations of The
Short Papers and Notes 303 A TaxonomicStudy of RecentlyIntroduced Populations of the Banded Darter,Etheostoma zonale (Cope), in the Susquehanna River. Etheostoma zonale (Cope), the banded darter, has an who supplied banded darters from Pine Creek. extensive distribution in the Mississippi drainage from A sample of 20 adult specimens was obtained from the Louisiana and Mississippi in the south to Minnesota and mouth of Stony Creek, Dauphin County, Pennsylvania, New York in the north. It is readily collected in the (lower Susquehanna drainage, 42 to 56 mm standard Allegheny tributaries of western Pennsylvania. length), from Pine Creek, Lycoming County, Pennsylva- The banded darter was first reported in Little Pine nia (upper Susquehanna drainage, 42 to 60 mm), and Creek, Lycoming County, Susquehanna River drainage from French Creek, Crawford County, Pennsylvania (Atlantic slope) in Kneib (1972) who suggested a bait (Allegheny drainage, 38 to 55 mm). They were examined bucket introduction. Denoncourt et al. (1975) reported for cheek, breast and belly squamation as described in TABLE 1. Meristic data from samples of three populations of the banded darter, Etheostoma zonale from Stony Creek (lower Susquehanna River), from Pine Creek (upper Susquehanna River) and from French Creek (Allegheny River). Lateral line scales 42 43 44 45 46 47 48 49 50 51 k ANOVA (p = .05) Stony Creek 1 1 7 5 2 1 3 - -- 45.1 NS Pine Creek - 1 2 4 5 4 3 - - 1 46.2 French Creek - 2 3 6 3 - 4 2 - - 45.8 Scales above lateral line 4 5 6 R ANOVA (p = .05) Stony Creek 3 -

Restoring the Endangered Oyster Mussel (Epioblasma Capsaeformis) to the Upper Clinch River, Virginia: an Evaluation of Population Restoration Techniques Caitlin S
RESEARCH ARTICLE Restoring the endangered oyster mussel (Epioblasma capsaeformis) to the upper Clinch River, Virginia: an evaluation of population restoration techniques Caitlin S. Carey1,2,3,JessW.Jones4, Robert S. Butler5, Eric M. Hallerman6 From 2005 to 2011, the federally endangered freshwater mussel Epioblasma capsaeformis (oyster mussel) was reintroduced at three sites in the upper Clinch River, Virginia, using four release techniques. These release techniques were (1) translocation of adults (site 1, n = 1418), (2) release of laboratory-propagated sub-adults (site 1, n = 2851), (3) release of 8-week-old laboratory-propagated juveniles (site 2, n = 9501), and (4) release of artificially infested host fishes (site 3, n = 1116 host fishes). These restoration efforts provided a unique research opportunity to compare the effectiveness of techniques used to reestablish populations of extirpated and declining species. We evaluated the relative success of these four population restoration approaches via monitoring at each release site (2011–2012) using systematic 0.25-m2 quadrat sampling to estimate abundance and post-release survival. Abundances of translocated adult and laboratory-propagated sub-adult E. capsaeformis at site 1 ranged 577–645 and 1678–1700 individuals, respectively, signifying successful settlement and high post-release survival. Two untagged individuals (29.1 and 27.3 mm) were observed, indicating that recruitment is occurring at site 1. No E. capsaeformis were found at sites where 8-week-old laboratory-propagated juveniles (site 2) and artificially infested host fishes (site 3) were released. Our results indicate that translocations of adults and releases of laboratory-propagated sub-adults were the most effective population restoration techniques for E. -

Guiding Species Recovery Through Assessment of Spatial And
Guiding Species Recovery through Assessment of Spatial and Temporal Population Genetic Structure of Two Critically Endangered Freshwater Mussel Species (Bivalvia: Unionidae) Jess Walter Jones ( [email protected] ) United States Fish and Wildlife Service Timothy W. Lane Virginia Department of Game and Inland Fisheries N J Eric M. Hallerman Virginia Tech: Virginia Polytechnic Institute and State University Research Article Keywords: Freshwater mussels, Epioblasma brevidens, E. capsaeformis, endangered species, spatial and temporal genetic variation, effective population size, species recovery planning, conservation genetics Posted Date: March 16th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-282423/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/28 Abstract The Cumberlandian Combshell (Epioblasma brevidens) and Oyster Mussel (E. capsaeformis) are critically endangered freshwater mussel species native to the Tennessee and Cumberland River drainages, major tributaries of the Ohio River in the eastern United States. The Clinch River in northeastern Tennessee (TN) and southwestern Virginia (VA) harbors the only remaining stronghold population for either species, containing tens of thousands of individuals per species; however, a few smaller populations are still extant in other rivers. We collected and analyzed genetic data to assist with population restoration and recovery planning for both species. We used an 888 base-pair sequence of the mitochondrial NADH dehydrogenase 1 (ND1) gene and ten nuclear DNA microsatellite loci to assess patterns of genetic differentiation and diversity in populations at small and large spatial scales, and at a 9-year (2004 to 2013) temporal scale, which showed how quickly these populations can diverge from each other in a short time period. -

Freshwater Mussel Survey of Clinchport, Clinch River, Virginia: Augmentation Monitoring Site: 2006
Freshwater Mussel Survey of Clinchport, Clinch River, Virginia: Augmentation Monitoring Site: 2006 By: Nathan L. Eckert, Joe J. Ferraro, Michael J. Pinder, and Brian T. Watson Virginia Department of Game and Inland Fisheries Wildlife Diversity Division October 28th, 2008 Table of Contents Introduction....................................................................................................................... 4 Objective ............................................................................................................................ 5 Study Area ......................................................................................................................... 6 Methods.............................................................................................................................. 6 Results .............................................................................................................................. 10 Semi-quantitative .................................................................................................. 10 Quantitative........................................................................................................... 11 Qualitative............................................................................................................. 12 Incidental............................................................................................................... 12 Discussion........................................................................................................................ -

Underwater Observation and Habitat Utilization of Three Rare Darters
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 5-2010 Underwater observation and habitat utilization of three rare darters (Etheostoma cinereum, Percina burtoni, and Percina williamsi) in the Little River, Blount County, Tennessee Robert Trenton Jett University of Tennessee - Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Natural Resources and Conservation Commons Recommended Citation Jett, Robert Trenton, "Underwater observation and habitat utilization of three rare darters (Etheostoma cinereum, Percina burtoni, and Percina williamsi) in the Little River, Blount County, Tennessee. " Master's Thesis, University of Tennessee, 2010. https://trace.tennessee.edu/utk_gradthes/636 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Robert Trenton Jett entitled "Underwater observation and habitat utilization of three rare darters (Etheostoma cinereum, Percina burtoni, and Percina williamsi) in the Little River, Blount County, Tennessee." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Wildlife and Fisheries Science. James L. Wilson, Major Professor We have read this thesis and recommend its acceptance: David A. Etnier, Jason G. -

Comparative Growth, Reproduction, Habitat and Food Utilization Of
Conservation Biology Research Grant Program Nongame Wildlife Program Division of Ecological Services Minnesota Department of Natural Resources COMPARATIVE GROWTH, REPRODUCTION, HABITAT AND FOOD UTILIZATION 0F DARTERS Of THE ST. CROIX RIVER DRAINAGE FINAL REPORT Submitted to: Lee Ann Pfannmuller Nongame Wildlife Program Minnesota Department of Natural Resources Box 7, 500 Lafayette Road St. Paul, Minnesota 55146 Submitted by: Jay T. Hatch, Ph.D. Division of Science, Business, and Mathematics General College University of Minnesota 216 Pillsbury Dr. SE Minneapolis, MN 55455 February 24, 1986 Introduction One of the most abundant and ubiquitous groups of nongame fishes found in Minnesota is the darter group (Percidae: Etheostomatini). These lively and colorful fishes inhabit nearly all of our streams and rivers and many of our lakes (Eddy and Underhill, 1974). We know in general that darters play an important role in the trophic structure of stream ecosystems (Cummins 1980), and we know that some species are important indicators of general water quality (Gerking 1945; Smith 1971; Pflieger 1975; Burr 1980; Karr 1981). Yet, we know very little about the specific life histories of the darters of our state, and we know even less about how their resource utilization patterns change with changes in habitat and community structure. To date, only three life history studies have been cared out on Minnesota darter populations. Erickson (1977) studied the banded darter (Etheostoma zonale) in the Cannon River; Coon (1982) studied several aspects of the comparative ecology of the rainbow (E. coeruleum), fantail (E. flabellare) and Johnny (E. nigrum) darters in the Root River; and Hatch (1982, 1986) studied the gilt darter (Percina evides) in the St. -

Proceedings of the United States National Museum
Proceedings of the United States National Museum SMITHSONIAN INSTITUTION . WASHINGTON, D.C. Volume 119 1966 Number 3550 CATALOG OF TYPE SPECIMENS OF THE DARTERS (PISCES, PERCIDAE, ETHEOSTOMATINI) ^ By Bruce B. Collette and Leslie W. Knapp ^ Introduction The darters are a tribe of small freshwater fishes restricted to North America. Some 212 specific and subspecific names have been pro- posed for the approximately 100 vahd described species. About a dozen species await description. As part of a long-term study of these fishes, we have prepared this catalog of type specimens. We hope that our efforts will be of value in furthering systematic research and stabilizing the nomenclature of this most fascinating group of North American fishes. In preparing this catalog we have attempted to examine or at least to verify the location of the type specimens of all nominal forms of the three presently recognized genera of darters: Percina, Ammo- crypta, and Etheostoma. By type specimens we mean holotypes, lectotypes, syntypes, paralectotypes, and paratypes. Each form is ' Fifth paper in a series on the systematics of the Percidae by the senior author. 2 Collette: Assistant Laboratory Director, Bureau of Commercial Fisheries Ichthyological Laboratory, Division of Fishes, U.S. National Museum; Knapp: Supervisor in charge of vertebrates, Oceanographic Sorting Center, Smithsonian Institution. a 2 PROCEEDINGS OF THE NATIONAL MUSEUM vol. 119 listed in alphabetical order by the generic and specific name used in the original description. If subgeneric allocation was made in the original description, that name has been placed in parentheses between the genus and the species. Holotypes or lectotypes are listed before paratypes or paralectotypes. -

Native Freshwater Mussels
Native Freshwater Mussels January 2007 Fish and Wildlife Habitat Management Leaflet Number 46 Introduction Freshwater mussels belong to the phylum Mollusca, the second most diverse group of animals in the world in terms of number of described species. The phy- lum consists of approximately 100,000 freshwater, marine, and terrestrial species and includes mussels, snails, octopi, squid, as well as several other less fa- miliar groups. Although freshwater mussels are dis- tributed throughout the world, they reach their great- est diversity in North America, east of the Mississippi River. United States mussel populations have been in Virginia Department of Game and Inland Fisheries decline since the late 1800s for a number of reasons. Although freshwater mussels are found throughout Currently, nearly three-quarters of North America’s much of the world, they reach their greatest diversity native freshwater mussel species are considered en- in North America. dangered, threatened, or species of special concern, and some researchers believe that as many as 35 spe- cies (12%) are already extinct. >80 species The objective of this leaflet is to raise awareness 71–80 species about the decline of freshwater mussels in North 61–70 species America, their life history requirements, and the im- 51–60 species 41–50 species portant ecological role they play in aquatic habitats. 31–40 species In addition, this leaflet provides a number of practi- 21–30 species cal habitat management considerations to help pro- 11–20 species tect freshwater mussel populations. Freshwater mus- 1–10 species sels can also be referred to as freshwater clams or Adapted from presentation of Kevis S. -

Appendix K-1 – Endangered Species Habitat and Wildlife Technical Report
I-69 Ohio River Crossing Project Draft Environmental Impact Statement APPENDIX K-1 Endangered, Threatened, and Rare Species Habitat Assessment and Wildlife Technical Report Clarification Note for Central Alternative 1: Central Alternatives 1A and 1B as described in the DEIS are physically the same alternative. The only difference between them is that Central Alternative 1A would include tolls on both the new I-69 bridge and on the US 41 bridge. Central Alternative 1B would only include tolls on the new I-69 bridge. Any reference in this document to Central Alternative 1 applies to both Central Alternative 1A and Central Alternative 1B. Appendices October 15 , 2018 (1'$1*(5('7+5($7(1(' $1'5$5(63(&,(6+$%,7$7 $66(660(17 $1':,/'/,)( 7( CHNICAL 5(3257 I-69I-69 O OHIOHI O RRIVERIVER CCROSSINGROSS IN G PPROJECTROJ ECT Evansville, IN and Henderson, KY I N D O T Endangered, Threatened, and Rare Species Habitat Assessment and Wildlife Technical Report I-69 Ohio River Crossing Project Evansville, IN and Henderson, KY Prepared by: Stantec Consulting Services I-69 Ohio River Crossing Project ETR Species Habitat Assessment and Wildlife Technical Report TABLE OF CONTENTS CHAPTER 1 – INTRODUCTION ............................................................................. 1-1 West Alternative 1 ............................................................................................. 1-4 West Alternative 2 ............................................................................................. 1-6 Central Alternative 1 ....................................................................................... -

Purple Cat's Paw Pearlymussel (Epioblasma Obliquata Obliquata)
Purple Cat’s Paw Pearlymussel (Epioblasma obliquata obliquata) 5-Year Review: Summary and Evaluation U.S. Fish and Wildlife Service, Midwest Region Ecological Services Field Office Columbus, Ohio April 2015 Table of Contents 1.0 GENERAL INFORMATION .......................................................................................................... 1 1.1 Reviewers ..................................................................................................................................... 1 1.2 Methodology used to complete the review: ................................................................................. 1 1.3 Background: ................................................................................................................................. 1 2.0 REVIEW ANALYSIS ..................................................................................................................... 2 2.1 Application of the 1996 Distinct Population Segment (DPS) policy ........................................... 2 2.2 Recovery Criteria ......................................................................................................................... 2 2.3 Updated Information and Current Species Status ........................................................................ 6 2.4 Synthesis..................................................................................................................................... 10 3.0 RESULTS .....................................................................................................................................