Hypnosedatives for Anxiety, Insomnia and Muscle Spasm
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insomnia and Anxiety in Older People Sleeping Pills Are Usually Not the Best Solution
® Insomnia and anxiety in older people Sleeping pills are usually not the best solution lmost one-third of older people in the people who take one of United States take sleeping pills. These these medicines sleep medicines are also sometimes called only a little longer and A“sedative-hypnotics” or “tranquilizers.” They better than those who affect the brain and spinal cord. don’t take a medicine. Doctors prescribe some of these medicines Sleeping pills can for sleep problems. Some of these medicines have serious side effects. also can be used to treat other conditions, such All sedative-hypnotic medicines have special as anxiety or alcohol withdrawal. Sometimes, risks for older adults. Seniors are likely to be doctors also prescribe certain anti-depressants more sensitive to the medicines’ effects than for sleep, even though that’s not what they’re younger adults. And these medicines may designed to treat. stay in older people’s bodies longer. These Most older adults should first try to treat their medicines can cause confusion and memory insomnia without medicines. According to the problems that: American Geriatrics Society, there are safer and • Increase the risk of falls and hip fractures. better ways to improve sleep or reduce anxiety. These are common causes of hospital stays Here’s why: and death in older people. Sleeping pills may not help much. • Increase the risk of car accidents. Many ads say that sleeping pills help people get a full, restful night’s sleep. But studies show that this is not exactly true in real life. On average, The new “Z” medicines also have risks. -

Once-Daily Skeletal Muscle Relaxant in SA
NEWS Once-daily skeletal muscle relaxant in SA Myprocam (cyclobenzaprine with spine-related disorders and other 8. What are the classes of muscle a protective coating, and a polymer extended release [ER] sports injuries are excellent candidates relaxants? membrane that controls the rate of hydrochloride) is the most widely for Myprocam. Skeletal muscle relaxants (SMRs) are drug release. prescribed skeletal muscle a group of structurally unrelated drugs, The formulation of the drug relaxant in the US, is now available 4. What are the common as shown in Figure 1. These are divided ensures early systemic exposure in SA. Myprocam’s safety and efficacy indications for which Myprocam into two categories: to cyclobenzaprine, with a plasma has been shown in more than 20 clinical is used, and what are the common • Antispasticity agents: Used to concentration at four hours that trials. It was recently launched by causes of these ailments? treat muscle spasticity caused by is similar to that observed with Adcock Ingram nationally, making SA Low back pain, neck pain, sports traumatic neurologic injury, multiple cyclobenzaprine IR. the second country (only to the US) to injuries, muscle sprains and strains. sclerosis, and other conditions. However, in contrast to the bring the product to market. Common causes of these conditions • Antispasmodic agents: Used fluctuating peaks and troughs in Myprocam, indicated for the relief of include sports injuries, car accidents, to treat muscular pain or spasm plasma cyclobenzaprine concentration muscle spasm associated with acute work-related injuries, and everyday associated with acute, nonspecific after administration of the IR and painful musculoskeletal conditions, activities, which account for acute musculoskeletal conditions. -

Uso Adecuado De BZD En Insomnio Y Ansiedad.Pdf
Vol. 6 Nº 1 · OCTUBRE 2014 BOLETÍN CANARIO DE USO RACIONAL DEL MEDICAMENTO DEL SCS Uso adecuado de BENZODIAZEPINAS en insomnio y ansiedad. SUMARIO PERFIL FARMACOLÓGICO DE LAS BZD (Tabla 1). - INTRODUCCIÓN 1 - PERFIL FARMACOLÓGICO DE LAS BENZODIAZEPINAS 1 No todas las BZD son iguales, ni tienen las mismas indicaciones. - BENZODIAZEPINAS EN EL INSOMNIO 2 Conocer algunos aspectos sobre su perfil farmacológico es esencial para realizar una prescripción adecuada y segura. - BENZODIAZEPINAS EN LA ANSIEDAD 4 - RECOMENDACIONES PARA SUSPENDER 5 Por lo general las BZD se absorben muy bien por vía oral, mientras TRATAMIENTOS CON BZD que la vía intramuscular presenta una absorción lenta e irregular, por - BIBLIOGRAFÍA 7 lo que no suele ser muy recomendada. En situaciones de emergencia (convulsiones) es preferible utilizar la vía endovenosa. INTRODUCCIÓN Inicio de acción y vida media: el inicio de acción es distinto según el principio activo y constituye un criterio fundamental en la selección de Las benzodiazepinas (BZD) son psicofármacos que actúan aumentan- las BZD. Puede ser: de inicio rápido (0,5-1 h), de utilidad en el insomnio do la acción del ácido gammaaminobutírico (GABA), principal neuro- de conciliación y en crisis de ansiedad; de inicio intermedio (1-3 h) o transmisor inhibidor del sistema nervioso central. Tienen indicaciones de inicio lento (> 3 h), preferibles en insomnio de mantenimiento o terapéuticas diversas, y auque su uso más habitual es en el tratamien- despertar precoz y en la ansiedad generalizada. to de la ansiedad e insomnio, también se utilizan en la inducción a la anestesia, en el tratamiento de las crisis comiciales, en el síndrome La vida media de las BZD es otro de los criterios de selección y puede 5 de abstinencia alcohólica, como tratamiento coadyuvante de de dolor ser : corta (< 6 h); intermedia (6-24 h) y larga (> 24 h). -

Early Morning Insomnia, Daytime Anxiety, and Organic Mental Disorder Associated with Triazolam
Early Morning Insomnia, Daytime Anxiety, and Organic Mental Disorder Associated with Triazolam Tjiauw-Ling Tan, MD, Edward 0. Bixler, PhD, Anthony Kales, MD, Roger J. Cadieux, MD, and Amy L. Goodman, MD Hershey, Pennsylvania A psychiatric syndrome characterized by agita Sleep Disorders Clinic. He began taking triazolam tion, paranoid ideation, depersonalization, and de at bedtime in a 0.5-mg dose eight months before pression, as well as paresthesias and hyperacusis, his referral. Although the drug was effective ini has been attributed to administration of triazolam tially, tolerance developed, causing the patient to (Halcion).1 The occurrence of these reactions led gradually increase the dosage until eventually he to the removal of the drug from the market in the was taking a total of 1.5 mg nightly. Netherlands. Isolated behavioral side effects that The physical examination revealed no contribu include amnesia2-4 and hallucinations5 have also tory conditions. However, assessment of the pa been reported with administration of triazolam. tient’s mental status revealed that he was extreme Rebound insomnia6 and early morning insom ly guarded and suspicious and preoccupied with nia,7 both associated with increases in daytime his sleeplessness to the degree that this hypochon anxiety,7,8 are withdrawal syndromes known driacal concern had a delusional quality. He also to occur with rapidly eliminated benzodiazepine described two episodes indicating memory impair hypnotics such as triazolam. Rebound insomnia ment; both incidents occurred in the late afternoon consists of a marked increase in wakefulness and involved preparing to eat certain foods, which above baseline levels following drug withdrawal. -

The In¯Uence of Medication on Erectile Function
International Journal of Impotence Research (1997) 9, 17±26 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 The in¯uence of medication on erectile function W Meinhardt1, RF Kropman2, P Vermeij3, AAB Lycklama aÁ Nijeholt4 and J Zwartendijk4 1Department of Urology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; 2Department of Urology, Leyenburg Hospital, Leyweg 275, 2545 CH The Hague, The Netherlands; 3Pharmacy; and 4Department of Urology, Leiden University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands Keywords: impotence; side-effect; antipsychotic; antihypertensive; physiology; erectile function Introduction stopped their antihypertensive treatment over a ®ve year period, because of side-effects on sexual function.5 In the drug registration procedures sexual Several physiological mechanisms are involved in function is not a major issue. This means that erectile function. A negative in¯uence of prescrip- knowledge of the problem is mainly dependent on tion-drugs on these mechanisms will not always case reports and the lists from side effect registries.6±8 come to the attention of the clinician, whereas a Another way of looking at the problem is drug causing priapism will rarely escape the atten- combining available data on mechanisms of action tion. of drugs with the knowledge of the physiological When erectile function is in¯uenced in a negative mechanisms involved in erectile function. The way compensation may occur. For example, age- advantage of this approach is that remedies may related penile sensory disorders may be compen- evolve from it. sated for by extra stimulation.1 Diminished in¯ux of In this paper we will discuss the subject in the blood will lead to a slower onset of the erection, but following order: may be accepted. -

GABA Receptors
D Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews Review No.7 / 1-2011 GABA receptors Wolfgang Froestl , CNS & Chemistry Expert, AC Immune SA, PSE Building B - EPFL, CH-1015 Lausanne, Phone: +41 21 693 91 43, FAX: +41 21 693 91 20, E-mail: [email protected] GABA Activation of the GABA A receptor leads to an influx of chloride GABA ( -aminobutyric acid; Figure 1) is the most important and ions and to a hyperpolarization of the membrane. 16 subunits with γ most abundant inhibitory neurotransmitter in the mammalian molecular weights between 50 and 65 kD have been identified brain 1,2 , where it was first discovered in 1950 3-5 . It is a small achiral so far, 6 subunits, 3 subunits, 3 subunits, and the , , α β γ δ ε θ molecule with molecular weight of 103 g/mol and high water solu - and subunits 8,9 . π bility. At 25°C one gram of water can dissolve 1.3 grams of GABA. 2 Such a hydrophilic molecule (log P = -2.13, PSA = 63.3 Å ) cannot In the meantime all GABA A receptor binding sites have been eluci - cross the blood brain barrier. It is produced in the brain by decarb- dated in great detail. The GABA site is located at the interface oxylation of L-glutamic acid by the enzyme glutamic acid decarb- between and subunits. Benzodiazepines interact with subunit α β oxylase (GAD, EC 4.1.1.15). It is a neutral amino acid with pK = combinations ( ) ( ) , which is the most abundant combi - 1 α1 2 β2 2 γ2 4.23 and pK = 10.43. -
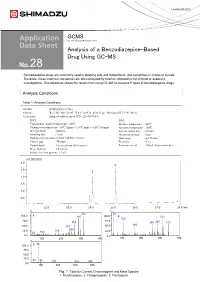
Analysis of a Benzodiazepine-Based Drug Using GC-MS 28
LAAN-E-MS-E028 GCMS Gas Chromatograph Mass Spectrometer Analysis of a Benzodiazepine-Based Drug Using GC-MS 28 Benzodiazepine drugs are commonly used in sleeping aids and tranquilizers, and sometimes in crimes or suicide. Therefore, these chemical substances are often analyzed by forensic laboratories for criminal or academic investigations. This datasheet shows the results from using GC-MS to measure 9 types of benzodiazepine drugs. Analysis Conditions Table 1: Analysis Conditions GC-MS :GCMS-QP2010 Ultra Column : Rxi®-5Sil MS (30 mL. X 0.25 mmI.D., df=0.25 µm, Shimadzu GLC P/N:13623) Glass insert :Silanized splitless insert (P/N: 221-48876-03) [GC] [MS] Vaporization chamber temperature : 260℃ Interface temperature : 280℃ Column oven temperature : 60℃ (2min) -> (10℃/min) -> 320℃ (10min) Ion source temperature : 200℃ Injection mode : Splitless Solvent elution time : 2.0 min Sampling time : 1 min Measurement mode : Scan High pressure injection method: 250 kPa (1.5 min) Mass range : m/z 35-600 Carrier gas : Helium Event time : 0.3 sec Control mode :Linear velocity (45.6 cm/sec) Emission current : 150 µA (high sensitivity) Purge flow rate :3.0 ml/min Sample injection quantity :1.0 µL (x1,000,000) 3.0 3 2.5 2 2.0 1.5 1.0 1 0.5 22.0 23.0 24.0 25.0 26.0 27.0 28.0 min % % 312 55 100.0 1 285 100.0 2 313 109 75.0 75.0 266 259287 342 166 50.0 238 50.0 248 25.0 183 25.0 63 109 0.0 0.0 100 200 300 400 100 200 300 400 % 86 100.0 3 75.0 50.0 25.0 58 99 183 315 387 0.0 100 200 300 400 Fig. -

Analytical Methods for Determination of Benzodiazepines. a Short Review
Cent. Eur. J. Chem. • 12(10) • 2014 • 994-1007 DOI: 10.2478/s11532-014-0551-1 Central European Journal of Chemistry Analytical methods for determination of benzodiazepines. A short review Review Article Paulina Szatkowska1, Marcin Koba1*, Piotr Kośliński1, Jacek Wandas1, Tomasz Bączek2,3 1Department of Toxicology, Faculty of Pharmacy, Collegium Medicum of Nicolaus Copernicus University, 85-089 Bydgoszcz, Poland 2Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Medical University of Gdańsk, 80-416 Gdańsk, Poland 3Institute of Health Sciences, Division of Human Anatomy and Physiology, Pomeranian University of Słupsk, 76-200 Słupsk, Poland Received 16 July 2013; Accepted 6 February 2014 Abstract: Benzodiazepines (BDZs) are generally commonly used as anxiolytic and/or hypnotic drugs as a ligand of the GABAA-benzodiazepine receptor. Moreover, some of benzodiazepines are widely used as an anti-depressive and sedative drugs, and also as anti-epileptic drugs and in some cases can be useful as an adjunct treatment in refractory epilepsies or anti-alcoholic therapy. High-performance liquid chromatography (HPLC) methods, thin-layer chromatography (TLC) methods, gas chromatography (GC) methods, capillary electrophoresis (CE) methods and some of spectrophotometric and spectrofluorometric methods were developed and have been extensively applied to the analysis of number of benzodiazepine derivative drugs (BDZs) providing reliable and accurate results. The available chemical methods for the determination of BDZs in biological materials and pharmaceutical formulations are reviewed in this work. Keywords: Analytical methods • Benzodiazepines • Drugs analysis • Pharmaceutical formulations © Versita Sp. z o.o. 1. Introduction and long). For this reason, an application of these drugs became broader allowing their utility to a larger extent, Benzodiazepines have been first introduced into medical and at the same time, problems related to drug abuse practice in the 60s of the last century. -

Comparison of Short-And Long-Acting Benzodiazepine-Receptor Agonists
J Pharmacol Sci 107, 277 – 284 (2008)3 Journal of Pharmacological Sciences ©2008 The Japanese Pharmacological Society Full Paper Comparison of Short- and Long-Acting Benzodiazepine-Receptor Agonists With Different Receptor Selectivity on Motor Coordination and Muscle Relaxation Following Thiopental-Induced Anesthesia in Mice Mamoru Tanaka1, Katsuya Suemaru1,2,*, Shinichi Watanabe1, Ranji Cui2, Bingjin Li2, and Hiroaki Araki1,2 1Division of Pharmacy, Ehime University Hospital, Shitsukawa, Toon, Ehime 791-0295, Japan 2Department of Clinical Pharmacology and Pharmacy, Neuroscience, Ehime University Graduate School of Medicine, Shitsukawa, Toon, Ehime 791-0295, Japan Received November 7, 2007; Accepted May 15, 2008 Abstract. In this study, we compared the effects of Type I benzodiazepine receptor–selective agonists (zolpidem, quazepam) and Type I/II non-selective agonists (zopiclone, triazolam, nitrazepam) with either an ultra-short action (zolpidem, zopiclone, triazolam) or long action (quazepam, nitrazepam) on motor coordination (rota-rod test) and muscle relaxation (traction test) following the recovery from thiopental-induced anesthesia (20 mg/kg) in ddY mice. Zolpidem (3 mg/kg), zopiclone (6 mg/kg), and triazolam (0.3 mg/kg) similarly caused an approximately 2-fold prolongation of the thiopental-induced anesthesia. Nitrazepam (1 mg/kg) and quazepam (3 mg/kg) showed a 6- or 10-fold prolongation of the anesthesia, respectively. Zolpidem and zopiclone had no effect on the rota-rod and traction test. Moreover, zolpidem did not affect motor coordination and caused no muscle relaxation following the recovery from the thiopental-induced anesthesia. However, zopiclone significantly impaired the motor coordination at the beginning of the recovery. Triazolam significantly impaired the motor coordination and muscle relaxant activity by itself, and these impairments were markedly exacerbated after the recovery from anesthesia. -

(12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness Et Al
USOO6264,917B1 (12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness et al. (45) Date of Patent: Jul. 24, 2001 (54) TARGETED ULTRASOUND CONTRAST 5,733,572 3/1998 Unger et al.. AGENTS 5,780,010 7/1998 Lanza et al. 5,846,517 12/1998 Unger .................................. 424/9.52 (75) Inventors: Jo Klaveness; Pál Rongved; Dagfinn 5,849,727 12/1998 Porter et al. ......................... 514/156 Lovhaug, all of Oslo (NO) 5,910,300 6/1999 Tournier et al. .................... 424/9.34 FOREIGN PATENT DOCUMENTS (73) Assignee: Nycomed Imaging AS, Oslo (NO) 2 145 SOS 4/1994 (CA). (*) Notice: Subject to any disclaimer, the term of this 19 626 530 1/1998 (DE). patent is extended or adjusted under 35 O 727 225 8/1996 (EP). U.S.C. 154(b) by 0 days. WO91/15244 10/1991 (WO). WO 93/20802 10/1993 (WO). WO 94/07539 4/1994 (WO). (21) Appl. No.: 08/958,993 WO 94/28873 12/1994 (WO). WO 94/28874 12/1994 (WO). (22) Filed: Oct. 28, 1997 WO95/03356 2/1995 (WO). WO95/03357 2/1995 (WO). Related U.S. Application Data WO95/07072 3/1995 (WO). (60) Provisional application No. 60/049.264, filed on Jun. 7, WO95/15118 6/1995 (WO). 1997, provisional application No. 60/049,265, filed on Jun. WO 96/39149 12/1996 (WO). 7, 1997, and provisional application No. 60/049.268, filed WO 96/40277 12/1996 (WO). on Jun. 7, 1997. WO 96/40285 12/1996 (WO). (30) Foreign Application Priority Data WO 96/41647 12/1996 (WO). -

Drug Screening: Actual Status, Pitfalls and Suggestions for Improvement Drogenscreening: Gegenwa¨ Rtiger Stand, Fehlermo¨ Glichkeiten Und Verbesserungsvorschla¨Ge
J Lab Med 2004;28(4):317–325 ᮊ 2004 by Walter de Gruyter • Berlin • New York 2004/03304 Drug Monitoring und Toxikologie Redaktion: V. W. Armstrong Drug screening: Actual status, pitfalls and suggestions for improvement Drogenscreening: Gegenwa¨ rtiger Stand, Fehlermo¨ glichkeiten und Verbesserungsvorschla¨ge Wolf R. Ku¨ lpmann* pretierbar. In Wirklichkeit mu¨ ssen viele Aspekte beru¨ ck- sichtigt werden, um einen aussagekra¨ ftigen Befund zu Klinische Chemie, Medizinische Hochschule Hannover, erhalten, z.B. Pra¨ analytik, Spezifita¨ t und Empfindlichkeit Hannover, Germany des Verfahrens oder Qualita¨ tssicherung. Obwohl die Abstract mechanisierten Meßverfahren naturgema¨ ß quantitative Ergebnisse liefern, wird aufgezeigt, daß es sich in der Immunoassays for drug screening are often regarded as Regel empfiehlt, qualitative Befunde zu erstellen. Die procedures which are easily performed and interpreted. Verfahren erlauben aber im Gegensatz zu den auch fu¨r But in fact, many aspects have to be considered to diese Zwecke verwendeten Teststreifen die Entschei- obtain a meaningful result. Among these are sampling, dungsgrenze (cut-off) der jeweiligen Fragestellung anzu- specificity and sensitivity of assays, and quality assess- passen, z.B. bei akuter Intoxikation, chronischem Abusus ment. Although the mechanised procedures yield quan- oder U¨ berwachung des Drogenentzugs. titative results, there are good reasons to generally report Ein schwerwiegender Nachteil von Immunoassays zum qualitative findings. The mechanised procedures allow Nachweis von Amphetamin und a¨ hnlichen Substanzen, the adjustment of the cut-off concentration to the clinical Barbituraten, Benzodiazepinen und Opiaten besteht dar- setting, e.g. in the case of acute intoxication, chronic in, daß eine starke Kreuzreaktivita¨ t des Antiko¨ rpers abuse or drug withdrawal, an important advantage as gegenu¨ ber pharmakologisch wenig wirksamen Verbin- compared to test strips. -

Medications in Long-Term Care: When Less Is More
Medications in Long-Term Care: When Less is More a, b,c Thomas W. Meeks, MD *, John W. Culberson, MD , d,e Monica S. Horton, MD, MSc KEYWORDS Long-term care Inappropriate prescribing Polypharmacy Psychotropics Opiates Sedatives HISTORY OF MEDICATION REDUCTION IN LONG-TERM CARE The Nursing Home Reform Act (OBRA-87) enacted in 1987 called for sweeping changes in the standards of care in nursing homes in accordance with new, more demanding federal regulations. For example, OBRA-87 called for a new approach to the use of antipsychotics in persons with dementia. Because antipsychotics were regarded as frequently inappropriate chemical restraints in long-term care (LTC), OBRA-87 mandated dose reductions in antipsychotics in an effort to discontinue them whenever possible. OBRA-87 proposed that a safer, more supportive environ- ment in LTC settings would facilitate such reductions in antipsychotic doses.1 Overall rates of potentially inappropriate prescribing in older adults have ranged from 12% to 40%, depending on the setting, criteria, and population sampled.2 Devel- oping from burgeoning concerns for polypharmacy and potential iatrogenic toxicity of medication in older adults, expert consensus lists of potentially inappropriate pharma- cotherapy in the elderly (PIPE) began to emerge. In general, drugs with activity in the central nervous system (CNS) were commonly placed on these PIPE lists, and hence our focus on such medications in this review. This work was supported by the following Geriatric Academic Career Awards: (1) K01 HP00047-02 (Dr. Meeks) (2) K01 HP00080-02 (Dr. Culberson) (3) K01 HP00114-02 (Dr. Horton). The authors have no conflicts of interest to disclose.