CYCLOBENZAPRINE (Brand Name: Flexeril®, Amrix®)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Once-Daily Skeletal Muscle Relaxant in SA
NEWS Once-daily skeletal muscle relaxant in SA Myprocam (cyclobenzaprine with spine-related disorders and other 8. What are the classes of muscle a protective coating, and a polymer extended release [ER] sports injuries are excellent candidates relaxants? membrane that controls the rate of hydrochloride) is the most widely for Myprocam. Skeletal muscle relaxants (SMRs) are drug release. prescribed skeletal muscle a group of structurally unrelated drugs, The formulation of the drug relaxant in the US, is now available 4. What are the common as shown in Figure 1. These are divided ensures early systemic exposure in SA. Myprocam’s safety and efficacy indications for which Myprocam into two categories: to cyclobenzaprine, with a plasma has been shown in more than 20 clinical is used, and what are the common • Antispasticity agents: Used to concentration at four hours that trials. It was recently launched by causes of these ailments? treat muscle spasticity caused by is similar to that observed with Adcock Ingram nationally, making SA Low back pain, neck pain, sports traumatic neurologic injury, multiple cyclobenzaprine IR. the second country (only to the US) to injuries, muscle sprains and strains. sclerosis, and other conditions. However, in contrast to the bring the product to market. Common causes of these conditions • Antispasmodic agents: Used fluctuating peaks and troughs in Myprocam, indicated for the relief of include sports injuries, car accidents, to treat muscular pain or spasm plasma cyclobenzaprine concentration muscle spasm associated with acute work-related injuries, and everyday associated with acute, nonspecific after administration of the IR and painful musculoskeletal conditions, activities, which account for acute musculoskeletal conditions. -

Management of Major Depressive Disorder Clinical Practice Guidelines May 2014
Federal Bureau of Prisons Management of Major Depressive Disorder Clinical Practice Guidelines May 2014 Table of Contents 1. Purpose ............................................................................................................................................. 1 2. Introduction ...................................................................................................................................... 1 Natural History ................................................................................................................................. 2 Special Considerations ...................................................................................................................... 2 3. Screening ........................................................................................................................................... 3 Screening Questions .......................................................................................................................... 3 Further Screening Methods................................................................................................................ 4 4. Diagnosis ........................................................................................................................................... 4 Depression: Three Levels of Severity ............................................................................................... 4 Clinical Interview and Documentation of Risk Assessment............................................................... -

Parkinson's Disease Fact Sheet
Parkinson’s Disease Fact Sheet About Parkinson’s Disease Parkinson’s disease is a progressive, incurable neurological disorder associated with a loss of dopamine-generating cells in the brain. It is primarily associated with progressive loss of motor control, but it results in a complex array of symptoms, including many non-motor symptoms. Parkinson’s impacts an estimated one million people in the United States. Critical Clinical Care Considerations • To avoid serious side effects, Parkinson’s patients need their medications on time, every time — do not skip or postpone doses. • Write down the exact times of day medications are to be administered so that doses are given on the same schedule the patient follows at home. • Do not substitute Parkinson’s medications or stop levodopa therapy abruptly. • Resume medications immediately following procedures, unless vomiting or severely incapacitated. • If an antipsychotic is necessary, use pimavanserin (Nuplazid), quetiapine (Seroquel) or clozapine (Clozaril). • Be alert for symptoms of dysphagia (trouble swallowing) and risk of pneumonia. • Ambulate as soon as medically safe. Patients may require assistance. Common Symptoms of Parkinson’s Disease Motor Non-Motor • Shaking or tremor at rest • Depression • Bradykinesia or freezing (being stuck • Anxiety in place when attempting to walk) • Constipation • Low voice volume or muffled speech • Cognitive decline and dementia • Lack of facial expression • Impulse control disorders • Stiffness or rigidity of the arms, legs • Orthostatic hypotension or -

Development and Validation of a LC-MS/MS Method for Detection and Quantification of 18 Antidepressants in Whole Blood
City University of New York (CUNY) CUNY Academic Works Student Theses John Jay College of Criminal Justice Spring 6-2019 Development and validation of a LC-MS/MS method for detection and quantification of 18 antidepressants in whole blood Linda Kim CUNY John Jay College, [email protected] How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/jj_etds/106 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Development and validation of a LC-MS/MS method for detection and quantification of 18 antidepressants in whole blood A Thesis Presented in Partial Fulfillment of the Requirements for the Degree of Master of Science in Forensic Science, John Jay College of Criminal Justice, City University of New York Linda Kim May 2019 Development and validation of a LC-MS/MS method for detection and quantification of 18 antidepressants in whole blood Linda Kim This Thesis has been presented to and accepted by the Office of Graduate Studies, John Jay College of Criminal Justice in Partial Fulfillment of the Requirements for the Degree of Master of Science in Forensic Science. Thesis Committee Thesis Advisor: Marta Concheiro-Guisan Second Reader: Shu-Yuan Cheng External Reader: Damon Borg i Acknowledgement I would like to thank Dr. Stripp for providing his laboratory for my research, and Dr. Borg and everyone in Cordant Laboratory for their guidance and support. I would like to thank Dr. Concheiro-Guisan for her time, patience, and information, and for making it possible to successfully complete this thesis. -

Medications in Long-Term Care: When Less Is More
Medications in Long-Term Care: When Less is More a, b,c Thomas W. Meeks, MD *, John W. Culberson, MD , d,e Monica S. Horton, MD, MSc KEYWORDS Long-term care Inappropriate prescribing Polypharmacy Psychotropics Opiates Sedatives HISTORY OF MEDICATION REDUCTION IN LONG-TERM CARE The Nursing Home Reform Act (OBRA-87) enacted in 1987 called for sweeping changes in the standards of care in nursing homes in accordance with new, more demanding federal regulations. For example, OBRA-87 called for a new approach to the use of antipsychotics in persons with dementia. Because antipsychotics were regarded as frequently inappropriate chemical restraints in long-term care (LTC), OBRA-87 mandated dose reductions in antipsychotics in an effort to discontinue them whenever possible. OBRA-87 proposed that a safer, more supportive environ- ment in LTC settings would facilitate such reductions in antipsychotic doses.1 Overall rates of potentially inappropriate prescribing in older adults have ranged from 12% to 40%, depending on the setting, criteria, and population sampled.2 Devel- oping from burgeoning concerns for polypharmacy and potential iatrogenic toxicity of medication in older adults, expert consensus lists of potentially inappropriate pharma- cotherapy in the elderly (PIPE) began to emerge. In general, drugs with activity in the central nervous system (CNS) were commonly placed on these PIPE lists, and hence our focus on such medications in this review. This work was supported by the following Geriatric Academic Career Awards: (1) K01 HP00047-02 (Dr. Meeks) (2) K01 HP00080-02 (Dr. Culberson) (3) K01 HP00114-02 (Dr. Horton). The authors have no conflicts of interest to disclose. -

Drug Class Review on Skeletal Muscle Relaxants
Drug Class Review on Skeletal Muscle Relaxants Final Report Update 2 May 2005 Original Report Date: April 2003 Update 1 Report Date: January 2004 A literature scan of this topic is done periodically The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Roger Chou, MD Kim Peterson, MS Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Copyright © 2005 by Oregon Health & Science University Portland, Oregon 97201. All rights reserved. Note: A scan of the medical literature relating to the topic is done periodically (see http://www.ohsu.edu/ohsuedu/research/policycenter/DERP/about/methods.cfm for scanning process description). Upon review of the last scan, the Drug Effectiveness Review Project governance group elected not to proceed with another full update of this report. Some portions of the report may not be up to date. Prior versions of this report can be accessed at the DERP website. Final Report Update 2 Drug Effectiveness Review Project TABLE OF CONTENTS Introduction........................................................................................................................4 Scope and -

Drug-Facilitated Sexual Assault Panel, Blood
DRUG-FACILITATED SEXUAL ASSAULT PANEL, BLOOD Blood Specimens (Order Code 70500) Alcohols Analgesics, cont. Anticonvulsants, cont. Antihistamines, cont. Ethanol Phenylbutazone Phenytoin Cyclizine Amphetamines Piroxicam Pregabalin Diphenhydramine Amphetamine Salicylic Acid* Primidone Doxylamine BDB Sulindac* Topiramate Fexofenadine Benzphetamine Tapentadol Zonisamide Guaifenesin Ephedrine Tizanidine Antidepressants Hydroxyzine MDA Tolmetin Amitriptyline Loratadine MDMA Tramadol Amoxapine Oxymetazoline* Mescaline* Anesthetics Bupropion Pyrilamine Methcathinone Benzocaine Citalopram Tetrahydrozoline Methamphetamine Bupivacaine Clomipramine Triprolidine Phentermine Etomidate Desipramine Antipsychotics PMA Ketamine Desmethylclomipramine 9-hydroxyrisperidone Phenylpropanolamine Lidocaine Dosulepin Aripiprazole Pseudoephedrine Mepivacaine Doxepin Buspirone Analgesics Methoxetamine Duloxetine Chlorpromazine Acetaminophen Midazolam Fluoxetine Clozapine Baclofen Norketamine Fluvoxamine Fluphenazine Buprenorphine Pramoxine* Imipramine Haloperidol Carisoprodol Procaine 1,3-chlorophenylpiperazine (mCPP) Mesoridazine Cyclobenzaprine Rocuronium Mianserin* Norclozapine Diclofenac Ropivacaine Mirtazapine Olanzapine Etodolac Antibiotics Nefazodone Perphenazine Fenoprofen Azithromycin* Nordoxepin Pimozide Hydroxychloroquine Chloramphenicol* Norfluoxetine Prochlorperazine Ibuprofen Ciprofloxacin* Norsertraline Quetiapine Ketoprofen Clindamycin* Nortriptyline Risperidone Ketorolac Erythromycin* Norvenlafaxine Thioridazine Meclofenamic Acid* Levofloxacin* Paroxetine -

Blood Thinners These Medications Require up to 2 Weeks of Discontinued Use Prior to a Myelogram Procedure
MEDICATIONS TO AVOID PRE & POST MYELOGRAMS Radiocontrast agents used in myelography have the ability to lower the seizure threshold and when used with other drugs that also have this ability, may lead to convulsions. Drugs that possess the ability to lower seizure threshold should be discontinued at least 48 hours prior to myelography if possible and should not be resumed until at least 24 hours post-procedure. Drugs are listed below. ***Always consult with your prescribing physician prior to stoppage of your medication. Phenothiazines Antidepressants MAO Inhibitors Acetophenazine (Tindal) Amitriptyline (Elavil) Furazolidone (Furoxone) Chlorpromazine (Thorazine) Amoxapine (Asendin) Isocarboxazid (Marplan) Promethazine (Phenergan) Bupropion *(Welbutrin, Zyban) Pargyline (Eutonyl) Ethopropazine (Parsidol) Clomipramine (Anafranil, Placil) Phenelzine (Nardil) Fluphenazine (Prolixin) Desipramine (Norapramin) Procarbazine (Matulane) Mesoridazine (Serentil) Doxepin (Sinequan) Tranlcypromine (Parnate) Methdilazine (Tacaryl) Imipramine (Tofranil) Perphenazine (Trilafon) Maprotiline (Ludiomil) CNS Stimulants Proclorperazine (Compazine) Nortriptyline (Pamelor) Amphetamines Promazine (Sparine) Protriptyline (Vivactil) Pemoline (Cylert) Promethazine (Phenergan) Trimipramine (Surmontil) Thiethylperazine (Torecan) Thioridazine (Mellaril) Combination Antidepressants Trifluoperazine (Stelazine) Amitriptyline + Chlordiazepoxide (Limbitrol DS) Triflupromazine (Vesprin) Amitriptyline + Perphenazine (Triavil) Largon Levoprome Miscellaneous Antipsychotics -
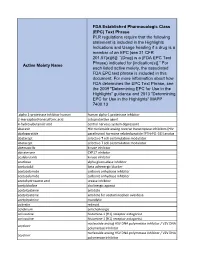
Active Moiety Name FDA Established Pharmacologic Class (EPC) Text
FDA Established Pharmacologic Class (EPC) Text Phrase PLR regulations require that the following statement is included in the Highlights Indications and Usage heading if a drug is a member of an EPC [see 21 CFR 201.57(a)(6)]: “(Drug) is a (FDA EPC Text Phrase) indicated for [indication(s)].” For Active Moiety Name each listed active moiety, the associated FDA EPC text phrase is included in this document. For more information about how FDA determines the EPC Text Phrase, see the 2009 "Determining EPC for Use in the Highlights" guidance and 2013 "Determining EPC for Use in the Highlights" MAPP 7400.13. .alpha. -

Anaesthetic Implications of Calcium Channel Blockers
436 Anaesthetic implications of calcium channel Leonard C. Jenkins aA MD CM FRCPC blockers Peter J. Scoates a sc MD FRCPC CONTENTS The object of this review is to emphasize the anaesthetic implications of calcium channel block- Physiology - calcium/calcium channel blockers Uses of calcium channel blockers ers for the practising anaesthetist. These drugs have Traditional played an expanding role in therapeutics since their Angina pectoris introduction and thus anaesthetists can expect to see Arrhythmias increasing numbers of patients presenting for anaes- Hypertension thesia who are being treated with calcium channel Newer and investigational Cardiac blockers. Other reviews have emphasized the basic - Hypertrophic cardiomyopathy pharmacology of calcium channel blockers. 1-7 - Cold cardioplegia - Pulmonary hypertension Physiology - calcium/calcium channel blockers Actions on platelets Calcium plays an important role in many physio- Asthma Obstetrics logical processes, such as blood coagulation, en- - Premature labor zyme systems, muscle contraction, bone metabo- - Pre-eclampsia lism, synaptic transmission, and cell membrane Achalasia and oesophageal spasm excitability. Especially important is the role of Increased intraocular pressure therapy calcium in myocardial contractility and conduction Protective effect on kidney after radiocontrast Cerebral vasospasm as well as in vascular smooth muscle reactivity. 7 Induced hypotensive anaesthesia Thus, it can be anticipated that any drug interfering Drag interactions with calcium channel blockers with the action of calcium could have widespread With anaesthetic agents effects. Inhalation agents In order to understand the importance of calcium - Effect on haemodynamics - Effect on MAC in cellular excitation, it is necessary to review some Neuromuscular blockers membrane physiology. Cell membranes are pri- Effects on epinephrine-induced arrhythmias marily phospholipids arranged in a bilayer. -

Paracetamol/Chlorzoxazone
PARACETAMOL/CHLORZOXAZONE TRADE NAME MYOLGIN™ NAME OF THE MEDICINAL PRODUCT Paracetamol / chlorzoxazone, 300 mg/250 mg, hard capsule QUALITATIVE AND QUANTITATIVE COMPOSITION Each hard capsule contains 300 mg of paracetamol and 250 mg of chlorzoxazone. Excipients Gelatin, Magnesium stearate, Talc. PHARMACEUTICAL FORM Hard gelatin capsules, purple opaque/pink printed Myolgin. CLINICAL INFORMATION Indications Paracetamol/chlorzoxazone is indicated for the treatment of: • sprains, strains, muscle pain, • traumatic muscle spasm, • reflex muscle spasm associated with rheumatic diseases, • lumbago, • myalgia, • tension headache, • torticollis, • cervical root syndrome, • rheumatoid arthritis. This medicinal product offers quick relief of pain and restore mobility even in severely painful spasms of the skeletal muscles in cases as above. Dosage and Administration Route of Administration For oral use. Adults Unless otherwise prescribed by a physician, the dose is 1 to 2 capsules three times daily after meals according to the severity of symptoms. Daily dose should not exceed 4 grams of paracetamol and 3 grams of chlorzoxazone. Do not exceed the stated dose. Minimum dosing interval: 4 hours Children This medicinal product is not recommended for children under 12 years. Elderly There are no relevant data available. Renal impairment Patients who have been diagnosed with renal impairment must seek medical advice before taking this medication (see Section Warnings and Precautions) Page 1 of 8 Hepatic impairment This medicinal product should not be given to patients with impaired liver function (see Section Warnings and Precautions). Contraindications Paracetamol / chlorzoxazone is contraindicated in: • hypersensitivity to the active substances or any of the excipients. Warnings and Precautions Renal impairment Care is advised in the administration of paracetamol/ chlorzoxazone to patients with renal impairment. -

Medications to Be Held for Allergy Skin Testing
Your appointment with Dr. Jill Poole, Dr. Sara May or Dr. Andrew Rorie Dr. Joel VanDeGraaff is scheduled for: _______________________ MEDICATIONS TO BE HELD FOR ALLERGY SKIN TESTING ANTIHISTAMINES (TO BE HELD FOR 5 DAYS): Clarinex (desloratidine) Claritin (loratidine) Allegra (fexofenadine) Chlor-Trimeton (chlorpheneramine) Dexchlorpheniramine Benadryl (diphenhydramine) Zyrtec (cetirizine) Xyzal (levocetirizine) Brovex (brompheniramine) Dimetapp Actifed Periactin (cyproheptadine) Drixoral (dexbrompheniramine) Please check your over the counter medications to see if they include an antihistamine EYE DROPS (TO BE HELD FOR 5 DAYS): Bepreve (bepotastine) Zaditor (ketotifen) Optivar (azelastine) Patanol/Pataday/Pazeo (olopatadine) All over the counter eye drops with antihistamine-A TOPICAL STEROID ANTI-INFLAMMATORIES (TO BE HELD FOR 5 DAYS): (Gels, Creams, Ointments, Solutions, and Lotions) ORAL PREDNISONE Ideally off oral steroids for two weeks; however, skin testing can be completed while on oral steroid use at less than 20 mg daily. ANTIDEPRESSANTS (TO BE HELD FOR 1-2 WEEKS AS APPROVED WITH PCP): Elavil (amitryptiline) Doxepin Trimipramine Desipramine Remeron (mirtazapine) Trazodone Serzone (nefazodone) Asendin (amoxapine) Pamelor (nortriptyline) Imipramine NASAL SPRAYS (TO BE HELD FOR 5 DAYS): Astelin (azelastine) Patanase (olopatadine) Astelin/Astepro (azelastine) Dymista HISTAMINE BLOCKERS (TO BE HELD FOR 1 DAY): Tagamet (cimetidine) Zantac (ranitidine) Axid (nizatidine) Pepcid (famotidine) OTHERS (TO BE HELD THE NIGHT BEFORE): Singulair (montelukast) Zyflo (zileuton) Accolate (zafirlukast) OTHERS (TO BE HELD 4-7 DAYS BEFORE): Vistaril/Atarax (hydroxyzine) Phenergan (promethazine) Xanax (alprazolam) Klonopin (clonazepam) Flexeril (cyclobenzaprine) Antivert/Bonine (meclizine) Tylenol Cold & Sinus *If you have any questions, please call 402-559-4015 and ask to speak to a Allergy nurse. .