Classification of Convolvulaceae: a Phylogenetic Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Macrophyte Structure in Lotic-Lentic Habitats from Brazilian Pantanal
Oecologia Australis 16(4): 782-796, Dezembro 2012 http://dx.doi.org/10.4257/oeco.2012.1604.05 MACROPHYTE STRUCTURE IN LOTIC-LENTIC HABITATS FROM BRAZILIAN PANTANAL Gisele Catian2*, Flávia Maria Leme2, Augusto Francener2, Fábia Silva de Carvalho2, Vitor Simão Galletti3, Arnildo Pott4, Vali Joana Pott4, Edna Scremin-Dias4 & Geraldo Alves Damasceno-Junior4 2Master, Program in Plant Biology, Federal University of Mato Grosso do Sul, Center for Biological Sciences and Health, Biology Department. Cidade Universitária, s/no – Caixa Postal: 549 – CEP: 79070-900, Campo Grande, MS, Brazil. 3Master, Program in Ecology and Conservation, Federal University of Mato Grosso do Sul, Center for Biological Sciences and Health, Biology Department. Cidade Universitária, s/no – Caixa Postal: 549 – CEP: 79070-900, Campo Grande, MS, Brazil. 4Lecturer, Program in Plant Biology, Federal University of Mato Grosso do Sul, Center for Biological Sciences and Health, Biology Department. Cidade Universitária, s/no – Caixa Postal: 549 – CEP: 79070-900, Campo Grande, MS, Brazil. E-mail: [email protected]*, [email protected], [email protected], [email protected], [email protected], arnildo. [email protected], [email protected], [email protected], [email protected] ABSTRACT The goal of this study was to compare the vegetation structure of macrophytes in an anabranch-lake system. Sampling was carried out at flood in three types of aquatic vegetation, (wild-rice, floating meadow and Polygonum bank) in anabranch Bonfim (lotic) and in lake Mandioré (lentic) in plots along transects, to estimate the percent coverage and record life forms of species. We collected 59 species in 50 genera and 28 families. -

The Evolution of Sexual Reproduction in Cuscuta (Convolvulaceae)
Wilfrid Laurier University Scholars Commons @ Laurier Theses and Dissertations (Comprehensive) 2011 The Evolution of Sexual Reproduction in Cuscuta (Convolvulaceae) Michael Wright Wilfrid Laurier University Follow this and additional works at: https://scholars.wlu.ca/etd Part of the Plant Breeding and Genetics Commons Recommended Citation Wright, Michael, "The Evolution of Sexual Reproduction in Cuscuta (Convolvulaceae)" (2011). Theses and Dissertations (Comprehensive). 1039. https://scholars.wlu.ca/etd/1039 This Thesis is brought to you for free and open access by Scholars Commons @ Laurier. It has been accepted for inclusion in Theses and Dissertations (Comprehensive) by an authorized administrator of Scholars Commons @ Laurier. For more information, please contact [email protected]. NOTE TO USERS This reproduction is the best copy available. UMI Library and Archives Bibliotheque et 1*1 Canada Archives Canada Published Heritage Direction du Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington OttawaONK1A0N4 OttawaONK1A0N4 Canada Canada Your file Votre reference ISBN: 978-0-494-75396-5 Our file Notre reference ISBN: 978-0-494-75396-5 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library and permettant a la Bibliotheque et Archives Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par I'lnternet, preter, telecommunication or on the Internet, distribuer et vendre des theses partout dans le loan, distribute and sell theses monde, a des fins commerciaies ou autres, sur worldwide, for commercial or non support microforme, papier, electronique et/ou commercial purposes, in microform, autres formats. -

The Native Vegetation of the Nattai and Bargo Reserves
The Native Vegetation of the Nattai and Bargo Reserves Project funded under the Central Directorate Parks and Wildlife Division Biodiversity Data Priorities Program Conservation Assessment and Data Unit Conservation Programs and Planning Branch, Metropolitan Environmental Protection and Regulation Division Department of Environment and Conservation ACKNOWLEDGMENTS CADU (Central) Manager Special thanks to: Julie Ravallion Nattai NP Area staff for providing general assistance as well as their knowledge of the CADU (Central) Bioregional Data Group area, especially: Raf Pedroza and Adrian Coordinator Johnstone. Daniel Connolly Citation CADU (Central) Flora Project Officer DEC (2004) The Native Vegetation of the Nattai Nathan Kearnes and Bargo Reserves. Unpublished Report. Department of Environment and Conservation, CADU (Central) GIS, Data Management and Hurstville. Database Coordinator This report was funded by the Central Peter Ewin Directorate Parks and Wildlife Division, Biodiversity Survey Priorities Program. Logistics and Survey Planning All photographs are held by DEC. To obtain a Nathan Kearnes copy please contact the Bioregional Data Group Coordinator, DEC Hurstville Field Surveyors David Thomas Cover Photos Teresa James Nathan Kearnes Feature Photo (Daniel Connolly) Daniel Connolly White-striped Freetail-bat (Michael Todd), Rock Peter Ewin Plate-Heath Mallee (DEC) Black Crevice-skink (David O’Connor) Aerial Photo Interpretation Tall Moist Blue Gum Forest (DEC) Ian Roberts (Nattai and Bargo, this report; Rainforest (DEC) Woronora, 2003; Western Sydney, 1999) Short-beaked Echidna (D. O’Connor) Bob Wilson (Warragamba, 2003) Grey Gum (Daniel Connolly) Pintech (Pty Ltd) Red-crowned Toadlet (Dave Hunter) Data Analysis ISBN 07313 6851 7 Nathan Kearnes Daniel Connolly Report Writing and Map Production Nathan Kearnes Daniel Connolly EXECUTIVE SUMMARY This report describes the distribution and composition of the native vegetation within and immediately surrounding Nattai National Park, Nattai State Conservation Area and Bargo State Conservation Area. -

Appendix Color Plates of Solanales Species
Appendix Color Plates of Solanales Species The first half of the color plates (Plates 1–8) shows a selection of phytochemically prominent solanaceous species, the second half (Plates 9–16) a selection of convol- vulaceous counterparts. The scientific name of the species in bold (for authorities see text and tables) may be followed (in brackets) by a frequently used though invalid synonym and/or a common name if existent. The next information refers to the habitus, origin/natural distribution, and – if applicable – cultivation. If more than one photograph is shown for a certain species there will be explanations for each of them. Finally, section numbers of the phytochemical Chapters 3–8 are given, where the respective species are discussed. The individually combined occurrence of sec- ondary metabolites from different structural classes characterizes every species. However, it has to be remembered that a small number of citations does not neces- sarily indicate a poorer secondary metabolism in a respective species compared with others; this may just be due to less studies being carried out. Solanaceae Plate 1a Anthocercis littorea (yellow tailflower): erect or rarely sprawling shrub (to 3 m); W- and SW-Australia; Sects. 3.1 / 3.4 Plate 1b, c Atropa belladonna (deadly nightshade): erect herbaceous perennial plant (to 1.5 m); Europe to central Asia (naturalized: N-USA; cultivated as a medicinal plant); b fruiting twig; c flowers, unripe (green) and ripe (black) berries; Sects. 3.1 / 3.3.2 / 3.4 / 3.5 / 6.5.2 / 7.5.1 / 7.7.2 / 7.7.4.3 Plate 1d Brugmansia versicolor (angel’s trumpet): shrub or small tree (to 5 m); tropical parts of Ecuador west of the Andes (cultivated as an ornamental in tropical and subtropical regions); Sect. -

Typification and Nomenclature of the Convolvulaceae in N. L. Burman's Flora Indica, with an Introduction to the Burman Collect
Candollea 60(2): 445-467 (2005) Typification and nomenclature of the Convolvulaceae in N. L. Burman’s Flora Indica, with an introduction to the Burman collection at Geneva GEORGES W. STAPLES & FERNAND JACQUEMOUD ABSTRACT STAPLES, G. W. & F. JACQUEMOUD (2005). Typification and nomenclature of the Convol- vulaceae in N. L. Burman’s Flora Indica, with an introduction to the Burman collection at Geneva. Candollea 60: 445-467. In English, English and French abstracts. The history and current status of the Burman Herbarium conserved at Geneva (G) are reviewed. Lectotypifications are made for seven Burman names in Convolvulaceae, Convolvulus angularis Burm. f., C. mollis Burm. f., C. nervosus Burm. f., C. uniflorus Burm. f., C. vitifolius Burm. f., Evolvulus emarginatus Burm. f., Ipomoea paniculata Burm. f., and an eighth name is neotypified, Porana volubilis Burm. f. A new lectotype for Convolvulus gemellus Burm. f. is selected. The discovery of the heretofore missing holotype of Ipomoea sagittifolia Burm. f. requires a name change for the widespread Old World species, I. sepiaria Roxb., which has recently undergone several name changes, latterly to I. marginata (Desr.) Manitz. RÉSUMÉ STAPLES, G. W. & F. JACQUEMOUD (2005). Typification et nomenclature des Convolvulaceae dans la Flora Indica de N. L. Burman, précédées d’une introduction aux collections Burman déposées à Genève. Candollea 60: 445-467. En anglais, résumés anglais et français. Les auteurs présentent l’histoire et la situation actuelle de l’herbier Burman conservé à Genève (G). Sept noms de la famille des Convolvulaceae publiés par Burman sont lectotypifiés, Convolvulus angularis Burm. f., C. mollis Burm. f., C. -

Aniseia Martinicensis (Jacq.) Choisy
Aniseia martinicensis (Jacq.) Choisy Identifiants : 2497/animar Association du Potager de mes/nos Rêves (https://lepotager-demesreves.fr) Fiche réalisée par Patrick Le Ménahèze Dernière modification le 05/10/2021 Classification phylogénétique : Clade : Angiospermes ; Clade : Dicotylédones vraies ; Clade : Astéridées ; Clade : Lamiidées ; Ordre : Solanales ; Famille : Convolvulaceae ; Classification/taxinomie traditionnelle : Règne : Plantae ; Sous-règne : Tracheobionta ; Division : Magnoliophyta ; Classe : Magnoliopsida ; Ordre : Solanales ; Famille : Convolvulaceae ; Genre : Aniseia ; Synonymes : Aniseia uniflora Choisy, Convolvulus martinicensis Jacq, Convolvulus uniflorus Burm.f, Ipomoea uniflora Roem. & Schult, Ipomoea uniflora Choisy, Ipomoea martinicensis G.F.W. Meyer, et d'autres ; Nom(s) anglais, local(aux) et/ou international(aux) : Ulan puteh, , Anndat trakuet, Lidah patong, Lotombo, Venthiruthali, Vor andatt trokourt ; Rapport de consommation et comestibilité/consommabilité inférée (partie(s) utilisable(s) et usage(s) alimentaire(s) correspondant(s)) : Parties comestibles : feuilles, légumes{{{0(+x) (traduction automatique) | Original : Leaves, Vegetable{{{0(+x) La plante entière est consommée en période de pénurie alimentaire. Les feuilles sont utilisées comme légume Partie testée : feuilles{{{0(+x) (traduction automatique) Original : Leaves{{{0(+x) Taux d'humidité Énergie (kj) Énergie (kcal) Protéines (g) Pro- Vitamines C (mg) Fer (mg) Zinc (mg) vitamines A (µg) 0 0 0 0 0 0 0 néant, inconnus ou indéterminés. Illustration(s) (photographie(s) et/ou dessin(s)): Page 1/3 Autres infos : dont infos de "FOOD PLANTS INTERNATIONAL" : Statut : C'est un aliment de famine{{{0(+x) (traduction automatique). Original : It is a famine food{{{0(+x). Distribution : Une plante tropicale. Cela peut être dans les marécages ou les forêts ouvertes. Il pousse près du niveau de la mer. C'est surtout près de la côte{{{0(+x) (traduction automatique). -

27April12acquatic Plants
International Plant Protection Convention Protecting the world’s plant resources from pests 01 2012 ENG Aquatic plants their uses and risks Implementation Review and Support System Support and Review Implementation A review of the global status of aquatic plants Aquatic plants their uses and risks A review of the global status of aquatic plants Ryan M. Wersal, Ph.D. & John D. Madsen, Ph.D. i The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of speciic companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned.All rights reserved. FAO encourages reproduction and dissemination of material in this information product. Non-commercial uses will be authorized free of charge, upon request. Reproduction for resale or other commercial purposes, including educational purposes, may incur fees. Applications for permission to reproduce or disseminate FAO copyright materials, and all queries concerning rights and licences, should be addressed by e-mail to [email protected] or to the Chief, Publishing Policy and Support Branch, Ofice of Knowledge Exchange, -

Pollen Evolution and Its Taxonomic Significance in Cuscuta (Dodders, Convolvulaceae)
Plant Syst Evol (2010) 285:83–101 DOI 10.1007/s00606-009-0259-4 ORIGINAL ARTICLE Pollen evolution and its taxonomic significance in Cuscuta (dodders, Convolvulaceae) Mark Welsh • Sasˇa Stefanovic´ • Mihai Costea Received: 12 June 2009 / Accepted: 28 December 2009 / Published online: 27 February 2010 Ó Springer-Verlag 2010 Abstract The pollen morphology of 148 taxa (135 spe- unknown in other Convolvulaceae, has evolved in Cuscuta cies and 13 varieties) of the parasitic plant genus Cuscuta only in two lineages (subg. Monogynella, and clade O of (dodders, Convolvulaceae) was examined using scanning subg. Grammica). Overall, the morphology of pollen electron microscopy. Six quantitative characters were supports Cuscuta as a sister to either the ‘‘bifid-style’’ coded using the gap-weighting method and optimized Convolvulaceae clade (Dicranostyloideae) or to one of the onto a consensus tree constructed from three large-scale members of this clade. Pollen characters alone are insuf- molecular phylogenies of the genus based on nuclear ficient to reconstruct phylogenetic relationships; however, internal transcribed spacer (ITS) and plastid trn-LF palynological information is useful for the species-level sequences. The results indicate that 3-zonocolpate pollen is taxonomy of Cuscuta. ancestral, while grains with more colpi (up to eight) have evolved only in two major lineages of Cuscuta (subg. Keywords Convolvulaceae Á Cuscuta Á Dodders Á Monogynella and clade O of subg. Grammica). Complex Evolution Á Phylogeny Á Pollen morphology Á morphological intergradations occur between species when Scanning electron microscopy Á Taxonomy their tectum is described using the traditional qualitative types—imperforate, perforate, and microreticulate. This continuous variation is better expressed quantitatively as Introduction ‘‘percent perforation,’’ namely the proportion of perforated area (puncta or lumina) from the total tectum surface. -

Convolvulaceae1
Photograph: Helen Owens © Department of Environment, Water and Natural Resources, Government of South Australia Department of All rights reserved Environment, Copyright of illustrations might reside with other institutions or Water and individuals. Please enquire for details. Natural Resources Contact: Dr Jürgen Kellermann Editor, Flora of South Australia (ed. 5) State Herbarium of South Australia PO Box 2732 Kent Town SA 5071 Australia email: [email protected] Flora of South Australia 5th Edition | Edited by Jürgen Kellermann CONVOLVULACEAE1 R.W. Johnson2 Annual or perennial herbs or shrubs, often with trailing or twining stems, or leafless parasites; leaves alternate, exstipulate. Inflorescence axillary, rarely terminal, cymose or reduced to a single flower; flowers regular, (4) 5 (6)-merous, bisexual; sepals free or rarely united, quincuncial; corolla sympetalous, funnel-shaped or campanulate, occasionally rotate or salver-shaped; stamens adnate to the base of the corolla, alternating with the corolla lobes, filaments usually flattened and dilated downwards; anthers 2-celled, dehiscing longitudinally; ovary superior, mostly 2-celled, occasionally with 1, 3 or 4 cells, subtended by a disk; ovules 2, rarely 1, in each cell; styles 1 or 2, stigmas variously shaped. Fruit capsular. About 58 genera and 1,650 species mainly tropical and subtropical; in Australia 20 genera, 1 endemic, with c. 160 species, 17 naturalised. The highly modified parasitic species of Cuscuta are sometimes placed in a separate family, the Cuscutaceae. 1. Yellowish leafless parasitic twiners ...................................................................................................................... 5. Cuscuta 1: Green leafy plants 2. Ovary distinctly 2-lobed; styles 2, inserted between the lobes of ovary (gynobasic style); leaves often kidney-shaped ............................................................................................................. -
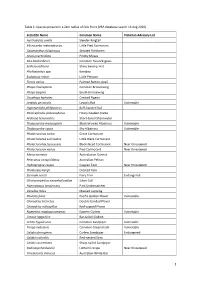
Scientific Name Common Name Victorian A
Table 1: Species present in a 2km radius of Crib Point (VBA database search 13 Aug 2020) Scientific Name Common Name Victorian Advisory List Austrolestes analis Slender Ringtail Microcarbo melanoleucos Little Pied Cormorant Calamanthus fuliginosus Striated Fieldwren Acacia verticillata Prickly Moses Poa labillardierei Common Tussock-grass Selliera radicans Shiny Swamp-mat Phyllostachys spp. Bamboo Eudyptula minor Little Penguin Turnix varius Painted Button-quail Phaps chalcoptera Common Bronzewing Phaps elegans Brush Bronzewing Ocyphaps lophotes Crested Pigeon Lewinia pectoralis Lewin's Rail Vulnerable Hypotaenidia philippensis Buff-banded Rail Poliocephalus poliocephalus Hoary-headed Grebe Ardenna tenuirostris Short-tailed Shearwater Thalassarche melanophris Black-browed Albatross Vulnerable Thalassarche cauta Shy Albatross Vulnerable Phalacrocorax carbo Great Cormorant Phalacrocorax sulcirostris Little Black Cormorant Phalacrocorax fuscescens Black-faced Cormorant Near threatened Phalacrocorax varius Pied Cormorant Near threatened Morus serrator Australasian Gannet Pelecanus conspicillatus Australian Pelican Hydroprogne caspia Caspian Tern Near threatened Thalasseus bergii Crested Tern Sternula nereis Fairy Tern Endangered Chroicocephalus novaehollandiae Silver Gull Haematopus longirostris Pied Oystercatcher Vanellus miles Masked Lapwing Pluvialis fulva Pacific Golden Plover Vulnerable Charadrius bicinctus Double-banded Plover Charadrius ruficapillus Red-capped Plover Numenius madagascariensis Eastern Curlew Vulnerable Limosa lapponica -

Larvicide of Aedes Aegypti (Diptera: Culicidae) from Ipomoea Pes-Caprae (Solanales: Convolvulaceae) Musri Musman, Sofyatuddin Karina, Said Almukhsin
AACL BIOFLUX Aquaculture, Aquarium, Conservation & Legislation International Journal of the Bioflux Society Larvicide of Aedes aegypti (Diptera: Culicidae) from Ipomoea pes-caprae (Solanales: Convolvulaceae) Musri Musman, Sofyatuddin Karina, Said Almukhsin Department of Marine Science, Marine and Fisheries Coordinatorate, Syiah Kuala University, Darussalam-Banda Aceh, Indonesia. Corresponding author: M. Musman, [email protected] Abstract. This research aimed to evaluate larvicidal candidate of the extracts of whole parts (roots, stems, leaves, flowers, and seeds) of Ipomoea pes-caprae (L.) R. Br. on Aedes aegypti (Linnaeus, 1762) larvae. The criteria applied to select larvicidal candidate were (1) the concentration of the extract solution must be ≤ 50 ppm, and (2) the larval mortality due to administration of the extract should be reached ≥ 75%. The I. pes-caprae parts were extracted with methanol and water solvents. Refer to the criteria, the methanol extract of the I. pes-caprae leaf was selected as the larvicidal candidate of the A. aegypti larvae. The 3rd instar of A. aegypti larvae was tested with five kinds of concentration of an aqueous solution of I. pes-caprae leaf extracts by completely random design with four replications. The methanol extract of I. pes-caprae leaf showed a very strong larvicide (LC50 was 12.60 ppm) of A. aegypti larvae. Key Words: larvae, Aedes aegypti, Ipomoea pes-caprae, instar, larvicidal candidate, methanol extract. Introduction. Dengue Hemorrhagic Fever (DHF) is a disease spread by the Aedes aegypti (Linnaeus, 1762) mosquito with a rapid rate of transmission and occurs in tropical regions, subtropical, and temperate in the whole world. DHF is one health problem in the world which the number of sufferers have been gradually increasing in quantity (Rao et al 2011). -

Solanales Nymphaeales Austrobaileyales
Amborellales Solanales Nymphaeales Austrobaileyales Acorales G Eenzaadlobbigen G Alismatales Solanales Petrosaviales Pandanales Van de Lamiiden (Garryales, Ge Dioscoreales Lamiales) is op basis van molecu Liliales morfologische kenmerken zeke Asparagales afstammelingen van één voorou Arecales Solanales is dat nog niet zeker, G Commeliniden G Dasypogonales verwantschappen tussen de fam Poales Commelinales In de samenstelling van de Sola Zingiberales ander veranderd. De Watergent (Menyanthaceae) is verplaatst n Ceratophyllales Vlambloemfamilie (Polemoniace Chloranthales de Bosliefjesfamilie (Hydrophyll Ruwbladigenfamilie (Boraginac Canellales Piperales de Montiniaceae uit de Ribesfam G Magnoliiden G Magnoliales Saxifragales), de Hydroleaceae u Laurales (was Boraginaceae), en de Sphe Ranunculales Klokjesfamilie (Campanulaceae, Sabiales Solanales hebben meestal versp Proteales bladeren zonder steunblaadjes. Trochodendrales Buxales regelmatig, met een vergroeidb en evenveel meeldraden als kro Gunnerales op de vrucht zitten. Iridoiden ko Berberidopsidales Dilleniales voor, maar wel allerlei alkaloide Caryophyllales Santalales Solanales Saxifragales Lamiids (Garryales, Gentianales, Sol G Geavanceerde tweezaadlobbigen G Vitales supposed to be monophyletic becau Crossosomatales anatomical, and morphological cha Geraniales of the order Solanales, and relation Myrtales are not yet clear. However, some ch in the composition of this order. Me Zygophyllales Celastrales moved to Asterales, Polemoniaceae Malpighiales Hydrophyllaceae to Boraginaceae.