From a Basic Microalga and an Acetic Acid Bacterium Cellulose Producer to a Living Symbiotic Biofilm
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Oh Ho Oh Oh Oh Oh O Ho Ch3o N N H O Ho Oh Oh Oh Oh O Ho Ch3o N N
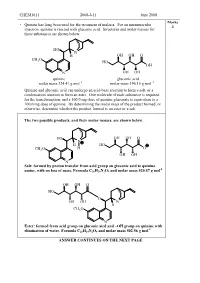
CHEM1611 2008-J-11 June 2008 Marks • Quinine has long been used for the treatment of malaria. For an intramuscular 4 injection, quinine is reacted with gluconic acid. Structures and molar masses for these substances are shown below. HO N H OH OH O CH O 3 HO OH N OH OH quinine gluconic acid molar mass 324.41 g mol –1 molar mass 196.16 g mol –1 Quinine and gluconic acid can undergo an acid-base reaction to form a salt, or a condensation reaction to form an ester. One molecule of each substance is required for the transformation, and a 160.0 mg dose of quinine gluconate is equivalent to a 100.0 mg dose of quinine. By determining the molar mass of the product formed, or otherwise, determine whether the product formed is an ester or a salt. The two possible products, and their molar masses, are shown below. HO OH OH O N H HO CH3O H O OH OH N Salt: formed by proton transfer from acid group on gluconic acid to quinine -1 amine, with no loss of mass. Formula C 26 H26 N2O9 and molar mass 520.57 g mol OH OH O HO O OH OH N H CH3O N Ester: formed from acid group on gluconic aicd and –OH group on quinine with -1 elimination of water. Formula C 26 H24 N2O8 and molar mass 502.56 g mol ANSWER CONTINUES ON THE NEXT PAGE CHEM1611 2008-J-11 June 2008 100.0 mg of quinine corresponds to: ̡̭̳̳ ̊̉̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.083 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̌̋̍.̍̊ ̧ ̭̯̬ ̊ 160.0 mg of the salt product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.074 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̋̉.̎̐ ̧ ̭̯̬ ̊ 160.0 mg of the ester product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.184 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̉̋.̎̏ ̧ ̭̯̬ ̊ As the dosages are the same, it must be the salt which is being administered. -

Embracing Bacterial Cellulose As a Catalyst for Sustainable Fashion
Running head: BACTERIAL CELLULOSE 1 Embracing Bacterial Cellulose as a Catalyst for Sustainable Fashion Luis Quijano A Senior Thesis submitted in partial fulfillment of the requirements for graduation in the Honors Program Liberty University Fall 2017 BACTERIAL CELLULOSE 2 Acceptance of Senior Honors Thesis This Senior Honors Thesis is accepted in partial fulfillment of the requirements for graduation from the Honors Program of Liberty University. ______________________________ Debbie Benoit, D.Min. Thesis Chair ______________________________ Randall Hubbard, Ph.D. Committee Member ______________________________ Matalie Howard, M.S. Committee Member ______________________________ David E. Schweitzer, Ph.D. Assistant Honors Director ______________________________ Date BACTERIAL CELLULOSE 3 Abstract Bacterial cellulose is a leather-like material produced during the production of Kombucha as a pellicle of bacterial cellulose (SCOBY) using Kombucha SCOBY, water, sugar, and green tea. Through an examination of the bacteria that produces the cellulose pellicle of the interface of the media and the air, currently named Komagataeibacter xylinus, an investigation of the growing process of bacterial cellulose and its uses, an analysis of bacterial cellulose’s properties, and a discussion of its prospects, one can fully grasp bacterial cellulose’s potential in becoming a catalyst for sustainable fashion. By laying the groundwork for further research to be conducted in bacterial cellulose’s applications as a textile, further commercialization of bacterial cellulose may become a practical reality. Keywords: Acetobacter xylinum, alternative textile, bacterial cellulose, fashion, Komagateibacter xylinus, sustainability BACTERIAL CELLULOSE 4 Embracing Bacterial Cellulose as a Catalyst for Sustainable Fashion Introduction One moment the fashionista is “in”, while the next day the fashionista is “out”. The fashion industry is typically known for its ever-changing trends, styles, and outfits. -

Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacte
S. Dinç et al. / Hacettepe J. Biol. & Chem., 2017, 45 (4), 603-607 Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacter Suşunun Glukonik Asit Üretiminde Kullanımı Research Article Saliha Dinç 1,2*, Meryem Kara2,3, Mehmet Öğüt3, Fatih Er1, Hacer Çiçekçi2 1Selcuk University Cumra School of Applied Sciences, Konya-Turkey. 2Selcuk Uni. Advanced Technology Research and Application Center, Konya-Turkey. 3Selcuk University Cumra Vocational High School, Konya-Turkey. ABSTRACT luconic acid, a food additive, is used in many foods to control acidity or binds metals such as calcium, Giron. Acinetobacter sp. WR326, newly isolated from soil possesses high phosphate solubilizing activity and do not require pyrroloquinoline quinone (PQQ) for glucose dehydrogenase (GDH) activity as cofactor In this study the gluconic acid production potential of this bacterium was investigated. Firstly, Acinetobacter sp. WR326 was incubated in tricalcium phosphate medium (TCP) with varying glucose concentrations (100, 250, 500 mM), at a temperature of 30°C for 5 days (120 hours). The highest gluconic acid yield (59%) was found at a glucose concentration of 100 mM. Then three different levels of gluconic acid addition to the medium (50, 100, 200 mM) were tested. When Acinetobacter sp. WR326 strain was cultivated with a 100 mM glucose and 100 mM gluconic acid the yield increased to 95.27%. In any trials 2 -keto D-gluconic acid, causes problems in processing and purification of the gluconic acid, was not detected in the medium. As a conclusion, Acinetobac- ter sp. WR326 may be considered novel potential bacterial strain for gluconic acid production. -

Cellulose Digestion and Metabolism Induced Biocatalytic Transitions in Anaerobic Microbial Ecosystems
Metabolites 2014, 4, 36-52; doi:10.3390/metabo4010036 OPEN ACCESS metabolites ISSN 2218-1989 www.mdpi.com/journal/metabolites/ Article Cellulose Digestion and Metabolism Induced Biocatalytic Transitions in Anaerobic Microbial Ecosystems Akira Yamazawa 1,2, Tomohiro Iikura 2, Yusuke Morioka 2, Amiu Shino 3, Yoshiyuki Ogata 4, Yasuhiro Date 2,3 and Jun Kikuchi 2,3,5,6,* 1 Research Planning and Management Group, Kajima Technical Research Institute, Kajima Corporation, 2-19-1 Tobitakyu, Chofu, Tokyo 182-0036, Japan; E-Mail: [email protected] 2 Graduate School of Medical Life Science, Yokohama City University, 1-7-29 Suehirocho, Tsurumi-ku, Yokohama, Kanagawa 230-0045, Japan; E-Mails: [email protected] (T.I.); [email protected] (Y.M.); [email protected] (Y.D.) 3 RIKEN Center for Sustainable Resource Science, 1-7-22 Suehirocho, Tsurumi-ku, Yokohama, Kanagawa 230-0045, Japan; E-Mail: [email protected] (A.S.) 4 Graduate School of Life and Environmental Sciences, Osaka Prefecture University, Osaka 599-8531, Japan; E-Mail: [email protected] 5 Graduate School of Bioagricultural Sciences, Nagoya University, 1 Furo-cho, Chikusa-ku, Nagoya, Aichi 464-0810, Japan 6 RIKEN Biomass Engineering Program, 2-1 Hirosawa, Wako 351-0198, Japan * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +81-45-503-9439; Fax: +81-45-503-9489. Received: 13 September 2013; in revised form: 18 December 2013 / Accepted: 20 December 2013 / Published: 31 December 2013 Abstract: Anaerobic digestion of highly polymerized biomass by microbial communities present in diverse microbial ecosystems is an indispensable metabolic process for biogeochemical cycling in nature and for industrial activities required to maintain a sustainable society. -

Bacterial Cellulose - Properties and Its Potential Application (Bakteria Selulosa - Sifat Dan Keupayaan Aplikasi)
Sains Malaysiana 50(2)(2021): 493-505 http://dx.doi.org/10.17576/jsm-2021-5002-20 Bacterial Cellulose - Properties and Its Potential Application (Bakteria Selulosa - Sifat dan Keupayaan Aplikasi) IZABELA BETLEJ, SARANI ZAKARIA, KRZYSZTOF J. KRAJEWSKI & PIOTR BORUSZEWSKI* ABSTRACT This review paper is related to the utilization on bacterial cellulose in many applications. The polymer produced from bacterial cellulose possessed a very good physical and mechanical properties, such as high tensile strength, elasticity, absorbency. The polymer from bacterial cellulose has a significantly higher degree of polymerization and crystallinity compared to those derived from plant. The collection of selected literature review shown that bacterial cellulose produced are in the form pure cellulose and can be used in many of applications. These include application in various industries and sectors of the economy, from medicine to paper or electronic industry. Keywords: Acetobacter xylinum; biocomposites; culturing; properties of bacterial cellulose ABSTRAK Ulasan kepustakaan ini adalah mengenai bakteria selulosa yang digunakan dalam banyak aplikasi. Bahan polimer yang terhasil daripada bakteria selulosa mempunyai sifat fizikal dan mekanikal yang sangat baik seperti sifat kekuatan regangan, kelenturan dan serapan. Bahan polimer terhasil daripada selulosa bakteria mempunyai darjah pempolimeran dan kehabluran yang tinggi berbanding daripada sumber tumbuhan. Suntingan kajian daripada beberapa koleksi ulasan kepustakaan menunjukkan bakteria selulosa terhasil adalah selulosa tulen yang boleh digunakan untuk banyak kegunaan. Antaranya adalah untuk pelbagai industri dan sektor ekonomi seperti perubatan atau industri elektronik. Kata kunci: Acetobacter xylinum; komposit-bio; pengkulturan; sifat bakteria selulosa INTRODUCTION Yamada et al. 2012). The first reports on the synthesis of Cellulose is the most common polymer found in nature. -

The Possibility of Recovering of Hydroxytyrosol from Olive Milling Wastewater by Enzymatic Bioconversion 265
ProvisionalChapter chapter 14 The PossibilityPossibility of of Recovering Recovering of Hydroxytyrosolof Hydroxytyrosol from fromOlive Milling Wastewater by Enzymatic Bioconversion Olive Milling Wastewater by Enzymatic Bioconversion Manel Hamza and Sami Sayadi Manel Hamza and Sami Sayadi Additional information is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/64774 Abstract This chapter discusses an innovative approach to obtain liquid fractions from olive mill wastewater (OMW) rich in hydroxytyrosol. The method is based on bioconversion combined with membrane separation techniques. An enzymatic bioconversion of three types of OMW was tested. TheS total volumes of OMW are 15 and 40 L. The reaction was monitored in mechanically stirred systems for 2 h at 50°C. Maximum hydroxytyr‐ osol concentrations of about 1.53, 0.83 and 0.46 g/L in the presence of 5 IU Aspergillus niger β‐glucosidase per milliliter from North OMW and South OMW were procured by two different olive millings, which are milling super press (MSP) and milling continuous chain (MCC), respectively. Enzymatic pretreatment was followed by two tangential flow membrane separation stages, microfiltration (MF) and ultrafiltration (UF). The ultrafiltration permeate was concentrated by evaporation at 45°C for 2 h. The latter exhibited a chemical oxygen demand (COD) level of 48.44 g/L. The UF permeate dehydration increased the hydroxytyrosol concentration to 7.2 g/L. A new natural product that contains some minerals beneficial to health and devoid of heavy metals or chemicals was obtained by this innovative work which describes an environmentally friendly process at pilot‐scale. -

Horizontal Operon Transfer, Plasmids, and the Evolution of Photosynthesis in Rhodobacteraceae
The ISME Journal (2018) 12:1994–2010 https://doi.org/10.1038/s41396-018-0150-9 ARTICLE Horizontal operon transfer, plasmids, and the evolution of photosynthesis in Rhodobacteraceae 1 2 3 4 1 Henner Brinkmann ● Markus Göker ● Michal Koblížek ● Irene Wagner-Döbler ● Jörn Petersen Received: 30 January 2018 / Revised: 23 April 2018 / Accepted: 26 April 2018 / Published online: 24 May 2018 © The Author(s) 2018. This article is published with open access Abstract The capacity for anoxygenic photosynthesis is scattered throughout the phylogeny of the Proteobacteria. Their photosynthesis genes are typically located in a so-called photosynthesis gene cluster (PGC). It is unclear (i) whether phototrophy is an ancestral trait that was frequently lost or (ii) whether it was acquired later by horizontal gene transfer. We investigated the evolution of phototrophy in 105 genome-sequenced Rhodobacteraceae and provide the first unequivocal evidence for the horizontal transfer of the PGC. The 33 concatenated core genes of the PGC formed a robust phylogenetic tree and the comparison with single-gene trees demonstrated the dominance of joint evolution. The PGC tree is, however, largely incongruent with the species tree and at least seven transfers of the PGC are required to reconcile both phylogenies. 1234567890();,: 1234567890();,: The origin of a derived branch containing the PGC of the model organism Rhodobacter capsulatus correlates with a diagnostic gene replacement of pufC by pufX. The PGC is located on plasmids in six of the analyzed genomes and its DnaA- like replication module was discovered at a conserved central position of the PGC. A scenario of plasmid-borne horizontal transfer of the PGC and its reintegration into the chromosome could explain the current distribution of phototrophy in Rhodobacteraceae. -

Wöhler Synthesis of Urea
Wöhler synthesis of urea Wöhler, 1928 – – + + NH Cl + K N C O 4 NH4 N C O O H2N NH2 Friedrich Wöhler Annalen der Physik und Chemie 1828, 88, 253–256 Significance: Wöhler was the first to make an organic substance from an inorganic substance. This was the beginning of the end of the theory of vitalism: the idea that organic and inorganic materials differed essentially by the presence of the “vital force”– present only in organic material. To his mentor Berzelius:“I can make urea without thereby needing to have kidneys, or anyhow, an animal, be it human or dog" Friedrich Wöhler, 1880-1882 Polytechnic School in Berlin Support for vitalism remained until 1845, when Kolbe synthesized acetic acid Polytechnic School at Kassel from carbon disulfide University of Göttingen Fischer synthesis of glucose PhHN N O N O NHPh Br aq. HCl Δ PhNH2NH2 HO H HO H O Br "α−acrose" H OH H OH H OH H OH CH2OH CH2OH α−acrosazone α−acrosone an “osazone” (osazone test for reducing sugars) Zn/AcOH OH OH CO2H CHO O HO H HO H OH Br2 HO H HO H Na-Hg HO H HNO3 HO H H OH H OH H OH H OH then resolve H O+ H OH via strychnine H OH H OH 3 H OH CH2OH salts CH2OH CH OH CH2OH 2 D-Mannonic acid DL-Mannose DL-Mannitol DL-Fructose quinoline • Established stereochemical relationship CO2H CHO H OH H OH between mannose and glucose Na-Hg HO H HO H (part of Fischer proof) H OH + H OH H3O • work mechanisms from acrosazone on H OH H OH Emil Fischer, 1852-1919 CH2OH CH2OH University of Munich (1875-81) D-Gluconic acid D-Glucose University of Erlangen (1881-88) University of Würzburg (1888-92) University of Berlin (1892-1919) Fischer, E. -

(Punica Granatum L.) Peel Waste: a Review
molecules Review Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review Tandokazi Pamela Magangana 1,2 , Nokwanda Pearl Makunga 1 , Olaniyi Amos Fawole 3 and Umezuruike Linus Opara 2,* 1 Department of Botany and Zoology, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa; [email protected] (T.P.M.); [email protected] (N.P.M.) 2 Postharvest Technology Research Laboratory, South African Research Chair in Postharvest Technology, Department of Horticultural Sciences, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Stellenbosch 7602, South Africa 3 Postharvest Research Laboratory, Department of Botany and Plant Biotechnology, University of Johannesburg, P.O. Box 524, Auckland Park, Johannesburg 2006, South Africa; [email protected] * Correspondence: [email protected]; Tel.: +27-21-808-4064 or +27-21-808-3743 Academic Editors: Ana Barros and Irene Gouvinhas Received: 5 September 2020; Accepted: 7 October 2020; Published: 14 October 2020 Abstract: Pomegranate peel has substantial amounts of phenolic compounds, such as hydrolysable tannins (punicalin, punicalagin, ellagic acid, and gallic acid), flavonoids (anthocyanins and catechins), and nutrients, which are responsible for its biological activity. However, during processing, the level of peel compounds can be significantly altered depending on the peel processing technique used, for example, ranging from 38.6 to 50.3 mg/g for punicalagins. This review focuses on the influence of postharvest processing factors on the pharmacological, phytochemical, and nutritional properties of pomegranate (Punica granatum L.) peel. Various peel drying strategies (sun drying, microwave drying, vacuum drying, and oven drying) and different extraction protocols (solvent, super-critical fluid, ultrasound-assisted, microwave-assisted, and pressurized liquid extractions) that are used to recover phytochemical compounds of the pomegranate peel are described. -

Print This Article
Macedonian Journal of Chemistry and Chemical Engineering, Vol. 38, No. 2, pp. 149–160 (2019) MJCCA9 – 775 ISSN 1857-5552 e-ISSN 1857-5625 Received: April 23, 2019 DOI: 10.20450/mjcce.2019.1775 Accepted: October 8, 2019 Original scientific paper IDENTIFICATION AND QUANTIFICATION OF PHENOLIC COMPOUNDS IN POMEGRANATE JUICES FROM EIGHT MACEDONIAN CULTIVARS Jasmina Petreska Stanoeva1,*, Nina Peneva1, Marina Stefova1, Viktor Gjamovski2 1Institute of Chemistry, Faculty of Natural Sciences and Mathematics, Ss Cyril and Methodius University, Skopje, Republic of Macedonia 2Institute of Agriculture, Ss Cyril and Methodius University, Skopje, Republic of Macedonia [email protected] Punica granatum L. is one of the species enjoying growing interest due to its complex and unique chemical composition that encompasses the presence of anthocyanins, ellagic acid and ellagitannins, gal- lic acid and gallotannins, proanthocyanidins, flavanols and lignans. This combination is deemed respon- sible for a wide range of health-promoting biological activities. This study was focused on the analysis of flavonoids, anthocyanins and phenolic acids in eight pomegranate varieties (Punica granatum) from Macedonia, in two consecutive years. Fruits from each cultivar were washed and manually peeled, and the juice was filtered. NaF (8.5 mg) was added to 100 ml juice as a stabilizer. The samples were centrifuged for 15 min at 3000 rpm and analyzed using an HPLC/DAD/MSn method that was optimized for determination of their polyphenolic fingerprints. The dominant anthocyanin in all pomegranate varieties was cyanidin-3-glucoside followed by cya- nidin and delphinidin 3,5-diglucoside. From the results, it can be concluded that the content of anthocyanins was higher in 2016 compared to 2017. -

Draft Genomic Sequence of Nereida Ignava CECT 5292T, a Marine Bacterium of the Family Rhodobacteraceae David R
Arahal et al. Standards in Genomic Sciences (2016) 11:21 DOI 10.1186/s40793-016-0141-2 SHORT GENOME REPORT Open Access Draft genomic sequence of Nereida ignava CECT 5292T, a marine bacterium of the family Rhodobacteraceae David R. Arahal*, María J. Pujalte and Lidia Rodrigo-Torres Abstract Nereida ignava strain 2SM4T (= CECT 5292T = DSM 16309T = CIP 108404T = CCUG 49433T) is a marine bacterium belonging to the Roseobacter group of the family Rhodobacteraceae within the class Alphaproteobacteria. The strain was isolated from sea water surrounding cultivated oysters 2–3 miles off the Mediterranean coast near Valencia (Spain) and was phylogenetically related to uncultured clones of gall symbiont bacteria of some species of Prionitis alga. Here we describe the genome sequence and annotation of this organism, the type strain of the single species of this genus. The genome comprised 2,888,349 bp, 2,872 protein-coding genes and 52 RNA genes. The annotation revealed the capacity to produce bacteriocins, vitamins and auxins. Besides, it contained sulfur cycling related genes. Keywords: Nereida ignava, Rhodobacteraceae, Mediterranean Sea, Strictly aerobic, Slightly halophilic, Marine bacteria Introduction of the red algal genus Prionitis [1]. Gall formation in this Nereida is a genus of the Roseobacter group, within the alga was reported to be induced by bacteria phylogenet- family Rhodobacteraceae, order Rhodobacterales, Class ically related to the Roseobacter group [6, 7]. It is known Alphaproteobacteria, so far containing only one species, that this relationship is species specific and coevolution Nereida ignava [1]. At the time of writing there are 197 exists between host and bacteria [8]. -

Catalytic Oxidation of Glucose with Hydrogen Peroxide and Colloidal Gold As Pseudo-Homogenous Catalyst
Chiang Mai J. Sci. 2016; 43(4) 825 Chiang Mai J. Sci. 2016; 43(4) : 825-833 http://epg.science.cmu.ac.th/ejournal/ Contributed Paper Catalytic Oxidation of Glucose with Hydrogen Peroxide and Colloidal Gold as Pseudo-Homogenous Catalyst: A Combined Experimental and Theoretical Investigation Jitrayut Jitonnom [a] and Christoph Sontag *[a] [a] Division of Chemistry, School of Science, University of Phayao, Phayao 56000, Thailand. *Author for correspondence; e-mail: [email protected] Received: 10 July 2013 Accepted: 25 April 2014 ABSTRACT Gold nanoparticles have been proved to act as oxidation catalyst for glucose oxidation, offering a “chemical” synthetic route to gluconic acid and gluconates - nowadays commercially produced by an enzyme catalyzed oxidation. Our investigations of the gold catalyzed oxidation route showed that gold nanoparticles produced by a modified Turkevich method have a high activity for this pseudo-homogenous catalytic reaction. Under mild reaction conditions, glucose could be oxidized in good yields (~70%) and the resulting gluconate could be isolated by column chromatography and precipitation as calcium salt. The catalytic oxidation reaction was found to follow the first-order kinetic with a rate constant of 4.95 h-1, in good agreement with previous finding. The underlying reaction mechanism is discussed, assuming that the formation of a gold-glucose cluster intermediate is a key catalytic step. Several structures of the gold-glucose intermediates were examined using density functional theory methods. The molecular behavior of glucose adsorption in gold colloid solution is present. Keywords: colloidal gold catalyst, glucose oxidation, gold nanoparticle, gluconic acid, DFT 1. INTRODUCTION Gold nanoparticles (Au NPs) as highly Catalysis by Au NPs has been emerged effective green catalysts for oxidative by the discovery of gold nanoparticles as processes have become a research topic of highly active supported catalysts for oxidation wide-spread interest in recent years [1-5], of CO into CO2 by atmospheric air [7].