Print This Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Oh Ho Oh Oh Oh Oh O Ho Ch3o N N H O Ho Oh Oh Oh Oh O Ho Ch3o N N
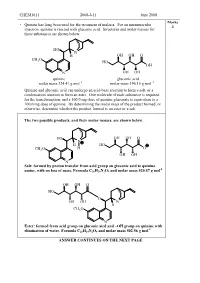
CHEM1611 2008-J-11 June 2008 Marks • Quinine has long been used for the treatment of malaria. For an intramuscular 4 injection, quinine is reacted with gluconic acid. Structures and molar masses for these substances are shown below. HO N H OH OH O CH O 3 HO OH N OH OH quinine gluconic acid molar mass 324.41 g mol –1 molar mass 196.16 g mol –1 Quinine and gluconic acid can undergo an acid-base reaction to form a salt, or a condensation reaction to form an ester. One molecule of each substance is required for the transformation, and a 160.0 mg dose of quinine gluconate is equivalent to a 100.0 mg dose of quinine. By determining the molar mass of the product formed, or otherwise, determine whether the product formed is an ester or a salt. The two possible products, and their molar masses, are shown below. HO OH OH O N H HO CH3O H O OH OH N Salt: formed by proton transfer from acid group on gluconic acid to quinine -1 amine, with no loss of mass. Formula C 26 H26 N2O9 and molar mass 520.57 g mol OH OH O HO O OH OH N H CH3O N Ester: formed from acid group on gluconic aicd and –OH group on quinine with -1 elimination of water. Formula C 26 H24 N2O8 and molar mass 502.56 g mol ANSWER CONTINUES ON THE NEXT PAGE CHEM1611 2008-J-11 June 2008 100.0 mg of quinine corresponds to: ̡̭̳̳ ̊̉̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.083 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̌̋̍.̍̊ ̧ ̭̯̬ ̊ 160.0 mg of the salt product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.074 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̋̉.̎̐ ̧ ̭̯̬ ̊ 160.0 mg of the ester product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.184 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̉̋.̎̏ ̧ ̭̯̬ ̊ As the dosages are the same, it must be the salt which is being administered. -

Walnut Polyphenol
ORYZA OIL & FAT CHEMICAL CO., L TD. WALNUT POLYPHENOL Hepatoprotective & Anti-oxidative Extract For Metabolic Syndrome ■ WALNUT POLYPHENOL-P10,P30 (Powder,Food Grade) ■ WALNUT POLYPHENOL-WSP10 (Water-soluble Powder,Food Grade) ■ WALNUT POLYPHENOL-PC10,PC30 (Powder,Cosmetic Grade) ■ WALNUT POLYPHENOL-WSPC10 (Water-soluble Powder,Cosmetic Grade) ■ WALNUT POLYPHENOL-LC (Water-soluble Liquid,Cosmetic Grade) ■ WALNUT SEED OIL (Oil,Food & Cosmetic Grade) ORYZA OIL & FAT CHEMICAL CO., LTD ver. 1.0 HS WALNUT POLYPHENOL ver.1.0 HS WALNUT POLYPHENOL Hepatoprotective & Anti-oxidative Extract For Metabolic Syndrome 1. Introduction Recently, there is an increased awareness on metabolic syndrome – a condition characterized by a group of metabolic risk factors in one person. They include abdominal obesity, atherogenic dyslipidemia, elevated blood pressure, insulin resistance, prothrombotic state & proinflammatory state. The dominant underlying risk factors appear to be abdominal obesity and insulin resistance. In addition, non-alcoholic fatty liver disease (NAFLD) is the most commonly associated “liver” manifestation of metabolic syndrome which can progress to advance liver disease (e.g. cirrhosis) with associated morbidity and mortality. Lifestyle therapies such as weight loss significantly improve all aspects of metabolic syndrome, as well as reducing progression of NAFLD and cardiovascular mortality. Walnut (Juglans regia L. seed) is one the most popular nuts consumed in the world. It is loaded in polyunsaturated fatty acids – linoleic acid (LA), oleic acid and α-linolenic acid (ALA), an ω3 fatty acid. It has been used since ancient times and epidemiological studies have revealed that incorporating walnuts in a healthy diet reduces the risk of cardiovascular diseases. Recent investigations reported that walnut diet improves the function of blood vessels and lower serum cholesterol. -

Punica Granatum L
Research Article Studies on antioxidant activity of red, white, and black pomegranate (Punica granatum L.) peel extract using DPPH radical scavenging method Uswatun Chasanah[1]* 1 Department of Pharmacy, Faculty of Health Science, University of Muhammadiyah Malangg, Malang, East Java, Indonesia * Corresponding Author’s Email: [email protected] ARTICLE INFO ABSTRACT Article History Pomegranate (Punica granatum L.) has high antioxidant activity. In Received September 1, 2020 Indonesia, there are red pomegranate, white pomegranate, and black Revised January 7, 2021 pomegranate. The purpose of this study was to determine the antioxidant Accepted January 14, 2021 activity of red pomegranate peel extract, white pomegranate peel extract, Published February 1, 2021 and black pomegranate peel extract. The extracts prepared by ultrasonic maceration in 96% ethanol, then evaporated until thick extract was Keywords obtained and its antioxidant activity was determined using the DPPH Antioxidant radical scavenging method. This study showed that all pomegranate peel Black pomegranate extract varieties have potent antioxidant activity and the black Red pomegranate pomegranate peel extract has the highest antioxidant power. White pomegranate Peel extract DPPH Doi 10.22219/farmasains.v5i2.13472 1. INTRODUCTION Pomegranate (Punica granatum L.) belongs to the Puricaceae family, a plant originating from the Middle East (Rana, Narzary & Ranade, 2010). All parts of the pomegranate, such as fruit (fruit juice, fruit seeds, peel fruit), leaves, flowers, roots, and bark, have therapeutic effects such as neuroprotective, antioxidant, repair vascular damage, and anti-inflammatory. The clinical application of this plant used in cancers, atherosclerosis, hyperlipidemia, carotid artery stenosis, myocardial perfusion, periodontal disease, bacterial infections, ultraviolet radiation, erectile dysfunction, male infertility, neonatal hypoxic-ischemic brain injury, Alzheimer's disease, and obesity (Jurenka, 2008; Mackler, Heber & Cooper, 2013). -

A Review on Antihyperglycemic and Antihepatoprotective Activity of Eco-Friendly Punica Granatum Peel Waste
Hindawi Publishing Corporation Evidence-Based Complementary and Alternative Medicine Volume 2013, Article ID 656172, 10 pages http://dx.doi.org/10.1155/2013/656172 Review Article A Review on Antihyperglycemic and Antihepatoprotective Activity of Eco-Friendly Punica granatum Peel Waste Sushil Kumar Middha,1 Talambedu Usha,2 and Veena Pande1 1 Department of Biotechnology, Bhimtal Campus, Kumaun University, Nainital, Uttarakhand 263136, India 2 Department of Biotechnology & Biochemistry, Maharani Lakshmi Ammanni College for Women, Bangalore 560012, India Correspondence should be addressed to Veena Pande; veena [email protected] Received 28 December 2012; Revised 25 March 2013; Accepted 25 April 2013 Academic Editor: Edwin L. Cooper Copyright © 2013 Sushil Kumar Middha et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Over the past decade, pomegranate (Punica granatum) is entitled as a wonder fruit because of its voluminous pharmacological properties. In 1830, P. g ranatum fruit was first recognized in United States Pharmacopeia; the Philadelphia edition introduced the rind of the fruit, the New York edition the bark of the root and further 1890 edition the stem bark was introduced. There are significant efforts and progress made in establishing thepharmacological mechanisms of peel (pericarp or rind) and the individual constituents responsible for them. This review provides an insight on the phytochemical components that contribute too antihyperglycemic, hepatoprotective, antihyperlipidemic effect, and numerous other effects of wonderful, economic, and eco- friendly pomegranate peel extract (PP). 1. Introduction containing sacs packed with a fleshy, juicy, red or whitish pulp. -

Anti-Candida Targets and Cytotoxicity of Casuarinin Isolated from Plinia Cauliflora Leaves in a Bioactivity-Guided Study
Molecules 2013, 18, 8095-8108; doi:10.3390/molecules18078095 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Anti-Candida Targets and Cytotoxicity of Casuarinin Isolated from Plinia cauliflora Leaves in a Bioactivity-Guided Study Tatiana M. Souza-Moreira 1, Juliana A. Severi 1,†, Keunsook Lee 2, Kanya Preechasuth 2, Emerson Santos 1, Neil A. R. Gow 2, Carol A. Munro 2, Wagner Vilegas 3 and Rosemeire C. L. R. Pietro 1,* 1 Department of Drugs and Medicines, School of Pharmaceutical Sciences, UNESP-Univ Estadual Paulista, Rodovia Araraquara-Jau, km 1, Araraquara, 14801-902, São Paulo, Brazil; E-Mails: [email protected] (T.M.S.M.); [email protected] (J.A.S.); [email protected] (E.S.) 2 Department of Microbiology, Institute of Medical Sciences, University of Aberdeen, Foresterhill, Aberdeen, AB25 2ZD, Scotland, UK; E-Mails: [email protected] (K.L.); [email protected] (K.P.); [email protected] (N.A.R.G.); [email protected] (C.A.M.) 3 Department of Organic Chemistry, Institute of Chemistry, UNESP-Univ Estadual Paulista, R. Francisco Degni s/n, Araraquara, 14800-900, São Paulo, Brazil; E-Mail: [email protected] (W.V.) † Nowadays this author is at Department of Pharmacy and Nutrition, Centro de Ciências Agrárias, UFES-Univ Federal do Espírito Santo, Alto Universitário, Guararema, 29500-000, Alegre, ES, Brazil. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +55-16-3301-6965; Fax: +55-16-3301-6990. Received: 27 May 2013; in revised form: 5 July 2013 / Accepted: 5 July 2013 / Published: 9 July 2013 Abstract: In addition to the bio-guided investigation of the antifungal activity of Plinia cauliflora leaves against different Candida species, the major aim of the present study was the search for targets on the fungal cell. -

Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacte
S. Dinç et al. / Hacettepe J. Biol. & Chem., 2017, 45 (4), 603-607 Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacter Suşunun Glukonik Asit Üretiminde Kullanımı Research Article Saliha Dinç 1,2*, Meryem Kara2,3, Mehmet Öğüt3, Fatih Er1, Hacer Çiçekçi2 1Selcuk University Cumra School of Applied Sciences, Konya-Turkey. 2Selcuk Uni. Advanced Technology Research and Application Center, Konya-Turkey. 3Selcuk University Cumra Vocational High School, Konya-Turkey. ABSTRACT luconic acid, a food additive, is used in many foods to control acidity or binds metals such as calcium, Giron. Acinetobacter sp. WR326, newly isolated from soil possesses high phosphate solubilizing activity and do not require pyrroloquinoline quinone (PQQ) for glucose dehydrogenase (GDH) activity as cofactor In this study the gluconic acid production potential of this bacterium was investigated. Firstly, Acinetobacter sp. WR326 was incubated in tricalcium phosphate medium (TCP) with varying glucose concentrations (100, 250, 500 mM), at a temperature of 30°C for 5 days (120 hours). The highest gluconic acid yield (59%) was found at a glucose concentration of 100 mM. Then three different levels of gluconic acid addition to the medium (50, 100, 200 mM) were tested. When Acinetobacter sp. WR326 strain was cultivated with a 100 mM glucose and 100 mM gluconic acid the yield increased to 95.27%. In any trials 2 -keto D-gluconic acid, causes problems in processing and purification of the gluconic acid, was not detected in the medium. As a conclusion, Acinetobac- ter sp. WR326 may be considered novel potential bacterial strain for gluconic acid production. -

(12) United States Patent (10) Patent No.: US 7,919,636 B2 Seeram Et Al
USOO7919636B2 (12) United States Patent (10) Patent No.: US 7,919,636 B2 Seeram et al. (45) Date of Patent: Apr. 5, 2011 (54) PURIFICATIONS OF POMEGRANATE Aviram, M., et al., “Pomegranate juice consumption inhibits serum ELLAGTANNINS AND THEIR USES angiotensin converting enzyme activity and reduces systolic blood THEREOF pressure.” (2001) Atherosclerosis, 158: 195-198. Cerda, B., et al., “Evaluation of bioavailability and metabolism in the (75) Inventors: Navindra P. Seeram, Los Angeles, CA rat of punicalagin, an antioxidant polyphenol from pomegranate juice.” (2003) Eur, J. Nutr., 42:18-28. (US); David Heber, Los Angeles, CA Cerda, B., et al., “Repeated oral administration of high doses of the (US) pomegranate elagitannin punicalaginto rats for 37 days is not toxic.” (2003) J. Agric. Food Chem. 51:3493-3501. (73) Assignee: The Regents of the University of Doig, A., et al., “Isolation and structure elucidation of punicalagin, a California, Oakland, CA (US) toxic hydrolysable tannin, from Terminalia oblongata.” (1990) J. Chem. Soc. Perkin Trans. I, 2317-2321. (*) Notice: Subject to any disclaimer, the term of this El-Toumy, S., et al., “Two ellagitannins from Punica granatum patent is extended or adjusted under 35 heartwood.” (2002) Phytochemistry, 61:971-974. U.S.C. 154(b) by 248 days. Filippich, L., et al., “Hepatotoxic and nephrotoxic principles in Terminalia oblongata.” (1991) Research in Veterinary Science, (21) Appl. No.: 12/143,657 50:17O-177. Gil, M., et al., “Antioxidant activity of pomegranate juice and its (22) Filed: Jun. 20, 2008 relationship with phenolic composition and processing.” (2000) J. Agric. Food Chem., 48:4581-4589. -

The Possibility of Recovering of Hydroxytyrosol from Olive Milling Wastewater by Enzymatic Bioconversion 265
ProvisionalChapter chapter 14 The PossibilityPossibility of of Recovering Recovering of Hydroxytyrosolof Hydroxytyrosol from fromOlive Milling Wastewater by Enzymatic Bioconversion Olive Milling Wastewater by Enzymatic Bioconversion Manel Hamza and Sami Sayadi Manel Hamza and Sami Sayadi Additional information is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/64774 Abstract This chapter discusses an innovative approach to obtain liquid fractions from olive mill wastewater (OMW) rich in hydroxytyrosol. The method is based on bioconversion combined with membrane separation techniques. An enzymatic bioconversion of three types of OMW was tested. TheS total volumes of OMW are 15 and 40 L. The reaction was monitored in mechanically stirred systems for 2 h at 50°C. Maximum hydroxytyr‐ osol concentrations of about 1.53, 0.83 and 0.46 g/L in the presence of 5 IU Aspergillus niger β‐glucosidase per milliliter from North OMW and South OMW were procured by two different olive millings, which are milling super press (MSP) and milling continuous chain (MCC), respectively. Enzymatic pretreatment was followed by two tangential flow membrane separation stages, microfiltration (MF) and ultrafiltration (UF). The ultrafiltration permeate was concentrated by evaporation at 45°C for 2 h. The latter exhibited a chemical oxygen demand (COD) level of 48.44 g/L. The UF permeate dehydration increased the hydroxytyrosol concentration to 7.2 g/L. A new natural product that contains some minerals beneficial to health and devoid of heavy metals or chemicals was obtained by this innovative work which describes an environmentally friendly process at pilot‐scale. -

Wöhler Synthesis of Urea
Wöhler synthesis of urea Wöhler, 1928 – – + + NH Cl + K N C O 4 NH4 N C O O H2N NH2 Friedrich Wöhler Annalen der Physik und Chemie 1828, 88, 253–256 Significance: Wöhler was the first to make an organic substance from an inorganic substance. This was the beginning of the end of the theory of vitalism: the idea that organic and inorganic materials differed essentially by the presence of the “vital force”– present only in organic material. To his mentor Berzelius:“I can make urea without thereby needing to have kidneys, or anyhow, an animal, be it human or dog" Friedrich Wöhler, 1880-1882 Polytechnic School in Berlin Support for vitalism remained until 1845, when Kolbe synthesized acetic acid Polytechnic School at Kassel from carbon disulfide University of Göttingen Fischer synthesis of glucose PhHN N O N O NHPh Br aq. HCl Δ PhNH2NH2 HO H HO H O Br "α−acrose" H OH H OH H OH H OH CH2OH CH2OH α−acrosazone α−acrosone an “osazone” (osazone test for reducing sugars) Zn/AcOH OH OH CO2H CHO O HO H HO H OH Br2 HO H HO H Na-Hg HO H HNO3 HO H H OH H OH H OH H OH then resolve H O+ H OH via strychnine H OH H OH 3 H OH CH2OH salts CH2OH CH OH CH2OH 2 D-Mannonic acid DL-Mannose DL-Mannitol DL-Fructose quinoline • Established stereochemical relationship CO2H CHO H OH H OH between mannose and glucose Na-Hg HO H HO H (part of Fischer proof) H OH + H OH H3O • work mechanisms from acrosazone on H OH H OH Emil Fischer, 1852-1919 CH2OH CH2OH University of Munich (1875-81) D-Gluconic acid D-Glucose University of Erlangen (1881-88) University of Würzburg (1888-92) University of Berlin (1892-1919) Fischer, E. -

(Punica Granatum L.) Peel Waste: a Review
molecules Review Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review Tandokazi Pamela Magangana 1,2 , Nokwanda Pearl Makunga 1 , Olaniyi Amos Fawole 3 and Umezuruike Linus Opara 2,* 1 Department of Botany and Zoology, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa; [email protected] (T.P.M.); [email protected] (N.P.M.) 2 Postharvest Technology Research Laboratory, South African Research Chair in Postharvest Technology, Department of Horticultural Sciences, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Stellenbosch 7602, South Africa 3 Postharvest Research Laboratory, Department of Botany and Plant Biotechnology, University of Johannesburg, P.O. Box 524, Auckland Park, Johannesburg 2006, South Africa; [email protected] * Correspondence: [email protected]; Tel.: +27-21-808-4064 or +27-21-808-3743 Academic Editors: Ana Barros and Irene Gouvinhas Received: 5 September 2020; Accepted: 7 October 2020; Published: 14 October 2020 Abstract: Pomegranate peel has substantial amounts of phenolic compounds, such as hydrolysable tannins (punicalin, punicalagin, ellagic acid, and gallic acid), flavonoids (anthocyanins and catechins), and nutrients, which are responsible for its biological activity. However, during processing, the level of peel compounds can be significantly altered depending on the peel processing technique used, for example, ranging from 38.6 to 50.3 mg/g for punicalagins. This review focuses on the influence of postharvest processing factors on the pharmacological, phytochemical, and nutritional properties of pomegranate (Punica granatum L.) peel. Various peel drying strategies (sun drying, microwave drying, vacuum drying, and oven drying) and different extraction protocols (solvent, super-critical fluid, ultrasound-assisted, microwave-assisted, and pressurized liquid extractions) that are used to recover phytochemical compounds of the pomegranate peel are described. -

Catalytic Oxidation of Glucose with Hydrogen Peroxide and Colloidal Gold As Pseudo-Homogenous Catalyst
Chiang Mai J. Sci. 2016; 43(4) 825 Chiang Mai J. Sci. 2016; 43(4) : 825-833 http://epg.science.cmu.ac.th/ejournal/ Contributed Paper Catalytic Oxidation of Glucose with Hydrogen Peroxide and Colloidal Gold as Pseudo-Homogenous Catalyst: A Combined Experimental and Theoretical Investigation Jitrayut Jitonnom [a] and Christoph Sontag *[a] [a] Division of Chemistry, School of Science, University of Phayao, Phayao 56000, Thailand. *Author for correspondence; e-mail: [email protected] Received: 10 July 2013 Accepted: 25 April 2014 ABSTRACT Gold nanoparticles have been proved to act as oxidation catalyst for glucose oxidation, offering a “chemical” synthetic route to gluconic acid and gluconates - nowadays commercially produced by an enzyme catalyzed oxidation. Our investigations of the gold catalyzed oxidation route showed that gold nanoparticles produced by a modified Turkevich method have a high activity for this pseudo-homogenous catalytic reaction. Under mild reaction conditions, glucose could be oxidized in good yields (~70%) and the resulting gluconate could be isolated by column chromatography and precipitation as calcium salt. The catalytic oxidation reaction was found to follow the first-order kinetic with a rate constant of 4.95 h-1, in good agreement with previous finding. The underlying reaction mechanism is discussed, assuming that the formation of a gold-glucose cluster intermediate is a key catalytic step. Several structures of the gold-glucose intermediates were examined using density functional theory methods. The molecular behavior of glucose adsorption in gold colloid solution is present. Keywords: colloidal gold catalyst, glucose oxidation, gold nanoparticle, gluconic acid, DFT 1. INTRODUCTION Gold nanoparticles (Au NPs) as highly Catalysis by Au NPs has been emerged effective green catalysts for oxidative by the discovery of gold nanoparticles as processes have become a research topic of highly active supported catalysts for oxidation wide-spread interest in recent years [1-5], of CO into CO2 by atmospheric air [7]. -

1 Universidade Federal Do Rio De Janeiro Instituto De
UNIVERSIDADE FEDERAL DO RIO DE JANEIRO INSTITUTO DE QUÍMICA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIA DE ALIMENTOS Ana Beatriz Neves Martins DEVELOPMENT AND STABILITY OF JABUTICABA (MYRCIARIA JABOTICABA) JUICE OBTAINED BY STEAM EXTRACTION RIO DE JANEIRO 2018 1 Ana Beatriz Neves Martins DEVELOPMENT AND STABILITY OF JABUTICABA (MYRCIARIA JABOTICABA) JUICE OBTAINED BY STEAM EXTRACTION Dissertação de Mestrado apresentada ao Programa de Pós-graduação em Ciência de Alimentos do Instituto de Química, da Universidade Federal do Rio de Janeiro como parte dos requisitos necessários à obtenção do título de Mestre em Ciência de Alimentos. Orientadores: Prof.ª Mariana Costa Monteiro Prof. Daniel Perrone Moreira RIO DE JANEIRO 2018 2 3 Ana Beatriz Neves Martins DEVELOPMENT AND STABILITY OF JABUTICABA (MYRCIARIA JABOTICABA) JUICE OBTAINED BY STEAM EXTRACTION Dissertação de Mestrado apresentada ao Programa de Pós-graduação em Ciência de Alimentos do Instituto de Química, da Universidade Federal do Rio de Janeiro como parte dos requisitos necessários à obtenção do título de Mestre em Ciência de Alimentos. Aprovada por: ______________________________________________________ Presidente, Profª. Mariana Costa Monteiro, INJC/UFRJ ______________________________________________________ Profª. Maria Lúcia Mendes Lopes, INJC/UFRJ ______________________________________________________ Profª. Lourdes Maria Correa Cabral, EMPBRAPA RIO DE JANEIRO 2018 4 ACKNOLEDGEMENTS Ninguém passa por essa vida sem alguém pra dividir momentos, sorrisos ou choros. Então, se eu cheguei até aqui, foi porque jamais estive sozinha, e não poderia deixar de agradecer aqueles que estiveram comigo, fisicamente ou em pensamento. Primeiramente gostaria de agradecer aos meus pais, Claudia e Ricardo, por tudo. Pelo amor, pela amizade, pela incansável dedicação, pelos valores passados e por todo esforço pra que eu pudesse ter uma boa educação.