Advances in the Study of Cancer Metastasis and Calcium Signaling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Expression Gene Network Analyses Reveal Molecular Mechanisms And
www.nature.com/scientificreports OPEN Diferential expression and co- expression gene network analyses reveal molecular mechanisms and candidate biomarkers involved in breast muscle myopathies in chicken Eva Pampouille1,2, Christelle Hennequet-Antier1, Christophe Praud1, Amélie Juanchich1, Aurélien Brionne1, Estelle Godet1, Thierry Bordeau1, Fréderic Fagnoul2, Elisabeth Le Bihan-Duval1 & Cécile Berri1* The broiler industry is facing an increasing prevalence of breast myopathies, such as white striping (WS) and wooden breast (WB), and the precise aetiology of these occurrences remains poorly understood. To progress our understanding of the structural changes and molecular pathways involved in these myopathies, a transcriptomic analysis was performed using an 8 × 60 K Agilent chicken microarray and histological study. The study used pectoralis major muscles from three groups: slow-growing animals (n = 8), fast-growing animals visually free from defects (n = 8), or severely afected by both WS and WB (n = 8). In addition, a weighted correlation network analysis was performed to investigate the relationship between modules of co-expressed genes and histological traits. Functional analysis suggested that selection for fast growing and breast meat yield has progressively led to conditions favouring metabolic shifts towards alternative catabolic pathways to produce energy, leading to an adaptive response to oxidative stress and the frst signs of infammatory, regeneration and fbrosis processes. All these processes are intensifed in muscles afected by severe myopathies, in which new mechanisms related to cellular defences and remodelling seem also activated. Furthermore, our study opens new perspectives for myopathy diagnosis by highlighting fne histological phenotypes and genes whose expression was strongly correlated with defects. Te poultry industry relies on the production of fast-growing chickens, which are slaughtered at high weights and intended for cutting and processing. -

Cytokine Nomenclature
RayBiotech, Inc. The protein array pioneer company Cytokine Nomenclature Cytokine Name Official Full Name Genbank Related Names Symbol 4-1BB TNFRSF Tumor necrosis factor NP_001552 CD137, ILA, 4-1BB ligand receptor 9 receptor superfamily .2. member 9 6Ckine CCL21 6-Cysteine Chemokine NM_002989 Small-inducible cytokine A21, Beta chemokine exodus-2, Secondary lymphoid-tissue chemokine, SLC, SCYA21 ACE ACE Angiotensin-converting NP_000780 CD143, DCP, DCP1 enzyme .1. NP_690043 .1. ACE-2 ACE2 Angiotensin-converting NP_068576 ACE-related carboxypeptidase, enzyme 2 .1 Angiotensin-converting enzyme homolog ACTH ACTH Adrenocorticotropic NP_000930 POMC, Pro-opiomelanocortin, hormone .1. Corticotropin-lipotropin, NPP, NP_001030 Melanotropin gamma, Gamma- 333.1 MSH, Potential peptide, Corticotropin, Melanotropin alpha, Alpha-MSH, Corticotropin-like intermediary peptide, CLIP, Lipotropin beta, Beta-LPH, Lipotropin gamma, Gamma-LPH, Melanotropin beta, Beta-MSH, Beta-endorphin, Met-enkephalin ACTHR ACTHR Adrenocorticotropic NP_000520 Melanocortin receptor 2, MC2-R hormone receptor .1 Activin A INHBA Activin A NM_002192 Activin beta-A chain, Erythroid differentiation protein, EDF, INHBA Activin B INHBB Activin B NM_002193 Inhibin beta B chain, Activin beta-B chain Activin C INHBC Activin C NM005538 Inhibin, beta C Activin RIA ACVR1 Activin receptor type-1 NM_001105 Activin receptor type I, ACTR-I, Serine/threonine-protein kinase receptor R1, SKR1, Activin receptor-like kinase 2, ALK-2, TGF-B superfamily receptor type I, TSR-I, ACVRLK2 Activin RIB ACVR1B -

CD147 Knockdown Improves the Antitumor Efficacy of Trastuzumab in HER2-Positive Breast Cancer Cells
www.impactjournals.com/oncotarget/ Oncotarget, Vol. 7, No. 36 Research Paper CD147 knockdown improves the antitumor efficacy of trastuzumab in HER2-positive breast cancer cells Lijuan Xiong1,*, Li Ding2,*, Haoyong Ning3,*, Chenglin Wu1, Kaifei Fu1, Yuxiao Wang1, Yan Zhang4, Yan Liu2, Lijun Zhou1 1Central Laboratory, Navy General Hospital, Beijing 100048, P.R. China 2The Third School of Clinical Medicine, Southern Medical University, Guangzhou, Guangdong, 510630, P.R.China 3Department of Pathology, Navy General Hospital, Beijing 100048, P.R. China 4Department of Surgery, Navy General Hospital, Beijing 100048, P.R. China *These authors equally contributed to this work Correspondence to: Lijun Zhou, e-mail: [email protected] Keywords: CD147, HER2, breast cancer, antibody drug resistance/sensitivity, trastuzumab efficacy Received: January 13, 2016 Accepted: May 04, 2016 Published: June 23, 2016 ABSTRACT Trastuzumab is widely used in the clinical treatment of human epidermal growth factor receptor-2 (HER2)-positive breast cancer, but the patient response rate is low. CD147 stimulates cancer cell proliferation, migration, metastasis and differentiation and is involved in chemoresistance in many types of cancer cells. Whether CD147 alters the effect of trastuzumab on HER2-positive breast cancer cells has not been previously reported. Our study confirmed that CD147 suppression enhances the effects of trastuzumab both in vitro and in vivo. CD147 suppression increased the inhibitory rate of trastuzumab and cell apoptosis in SKBR3, BT474, HCC1954 and MDA- MB453 cells compared with the controls. Furthermore, CD147 knockdown increased expression of cleaved Caspase-3/9 and poly (ADP-ribose) polymerase (PARP) and decreased both mitogen-activated protein kinase (MAPK) and Akt phosphorylation in the four cell lines. -

Transient Receptor Potential (TRP) Channels in Haematological Malignancies: an Update
biomolecules Review Transient Receptor Potential (TRP) Channels in Haematological Malignancies: An Update Federica Maggi 1,2 , Maria Beatrice Morelli 2 , Massimo Nabissi 2 , Oliviero Marinelli 2 , Laura Zeppa 2, Cristina Aguzzi 2, Giorgio Santoni 2 and Consuelo Amantini 3,* 1 Department of Molecular Medicine, Sapienza University, 00185 Rome, Italy; [email protected] 2 Immunopathology Laboratory, School of Pharmacy, University of Camerino, 62032 Camerino, Italy; [email protected] (M.B.M.); [email protected] (M.N.); [email protected] (O.M.); [email protected] (L.Z.); [email protected] (C.A.); [email protected] (G.S.) 3 Immunopathology Laboratory, School of Biosciences and Veterinary Medicine, University of Camerino, 62032 Camerino, Italy * Correspondence: [email protected]; Tel.: +30-0737403312 Abstract: Transient receptor potential (TRP) channels are improving their importance in differ- ent cancers, becoming suitable as promising candidates for precision medicine. Their important contribution in calcium trafficking inside and outside cells is coming to light from many papers published so far. Encouraging results on the correlation between TRP and overall survival (OS) and progression-free survival (PFS) in cancer patients are available, and there are as many promising data from in vitro studies. For what concerns haematological malignancy, the role of TRPs is still not elucidated, and data regarding TRP channel expression have demonstrated great variability throughout blood cancer so far. Thus, the aim of this review is to highlight the most recent findings Citation: Maggi, F.; Morelli, M.B.; on TRP channels in leukaemia and lymphoma, demonstrating their important contribution in the Nabissi, M.; Marinelli, O.; Zeppa, L.; perspective of personalised therapies. -

Type of the Paper (Article
Table S1. Gene expression of pro-angiogenic factors in tumor lymph nodes of Ibtk+/+Eµ-myc and Ibtk+/-Eµ-myc mice. Fold p- Symbol Gene change value 0,007 Akt1 Thymoma viral proto-oncogene 1 1,8967 061 0,929 Ang Angiogenin, ribonuclease, RNase A family, 5 1,1159 481 0,000 Angpt1 Angiopoietin 1 4,3916 117 0,461 Angpt2 Angiopoietin 2 0,7478 625 0,258 Anpep Alanyl (membrane) aminopeptidase 1,1015 737 0,000 Bai1 Brain-specific angiogenesis inhibitor 1 4,0927 202 0,001 Ccl11 Chemokine (C-C motif) ligand 11 3,1381 149 0,000 Ccl2 Chemokine (C-C motif) ligand 2 2,8407 298 0,000 Cdh5 Cadherin 5 2,5849 744 0,000 Col18a1 Collagen, type XVIII, alpha 1 3,8568 388 0,003 Col4a3 Collagen, type IV, alpha 3 2,9031 327 0,000 Csf3 Colony stimulating factor 3 (granulocyte) 4,3332 258 0,693 Ctgf Connective tissue growth factor 1,0195 88 0,000 Cxcl1 Chemokine (C-X-C motif) ligand 1 2,67 21 0,067 Cxcl2 Chemokine (C-X-C motif) ligand 2 0,7507 631 0,000 Cxcl5 Chemokine (C-X-C motif) ligand 5 3,921 328 0,000 Edn1 Endothelin 1 3,9931 042 0,001 Efna1 Ephrin A1 1,6449 601 0,002 Efnb2 Ephrin B2 2,8858 042 0,000 Egf Epidermal growth factor 1,726 51 0,000 Eng Endoglin 0,2309 467 0,000 Epas1 Endothelial PAS domain protein 1 2,8421 764 0,000 Ephb4 Eph receptor B4 3,6334 035 V-erb-b2 erythroblastic leukemia viral oncogene homolog 2, 0,000 Erbb2 3,9377 neuro/glioblastoma derived oncogene homolog (avian) 024 0,000 F2 Coagulation factor II 3,8295 239 1 0,000 F3 Coagulation factor III 4,4195 293 0,002 Fgf1 Fibroblast growth factor 1 2,8198 748 0,000 Fgf2 Fibroblast growth factor -

Snapshot: Mammalian TRP Channels David E
SnapShot: Mammalian TRP Channels David E. Clapham HHMI, Children’s Hospital, Department of Neurobiology, Harvard Medical School, Boston, MA 02115, USA TRP Activators Inhibitors Putative Interacting Proteins Proposed Functions Activation potentiated by PLC pathways Gd, La TRPC4, TRPC5, calmodulin, TRPC3, Homodimer is a purported stretch-sensitive ion channel; form C1 TRPP1, IP3Rs, caveolin-1, PMCA heteromeric ion channels with TRPC4 or TRPC5 in neurons -/- Pheromone receptor mechanism? Calmodulin, IP3R3, Enkurin, TRPC6 TRPC2 mice respond abnormally to urine-based olfactory C2 cues; pheromone sensing 2+ Diacylglycerol, [Ca ]I, activation potentiated BTP2, flufenamate, Gd, La TRPC1, calmodulin, PLCβ, PLCγ, IP3R, Potential role in vasoregulation and airway regulation C3 by PLC pathways RyR, SERCA, caveolin-1, αSNAP, NCX1 La (100 µM), calmidazolium, activation [Ca2+] , 2-APB, niflumic acid, TRPC1, TRPC5, calmodulin, PLCβ, TRPC4-/- mice have abnormalities in endothelial-based vessel C4 i potentiated by PLC pathways DIDS, La (mM) NHERF1, IP3R permeability La (100 µM), activation potentiated by PLC 2-APB, flufenamate, La (mM) TRPC1, TRPC4, calmodulin, PLCβ, No phenotype yet reported in TRPC5-/- mice; potentially C5 pathways, nitric oxide NHERF1/2, ZO-1, IP3R regulates growth cones and neurite extension 2+ Diacylglycerol, [Ca ]I, 20-HETE, activation 2-APB, amiloride, Cd, La, Gd Calmodulin, TRPC3, TRPC7, FKBP12 Missense mutation in human focal segmental glomerulo- C6 potentiated by PLC pathways sclerosis (FSGS); abnormal vasoregulation in TRPC6-/- -
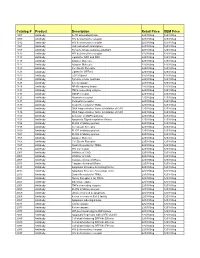
Comprehensive Product List
Catalog # Product Description Retail Price OEM Price 1007 Antibody IL-1R associated kinase 225/100ug 125/100ug 1009 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1012 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1021 Antibody JAK activated transcription 225/100ug 125/100ug 1107 Antibody Tyrosine kinase substrate p62DOK 225/100ug 125/100ug 1112 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1113 Antibody Ligand for DR4 and DR5 225/100ug 125/100ug 1115 Antibody Adapter Molecule 225/100ug 125/100ug 1117 Antibody Adapter Molecule 225/100ug 125/100ug 1120 Antibody Cell Death Receptor 225/100ug 125/100ug 1121 Antibody Ligand for GFRa-2 225/100ug 125/100ug 1123 Antibody CCR3 ligand 225/100ug 125/100ug 1125 Antibody Tyrosine kinase substrate 225/100ug 125/100ug 1128 Antibody A new caspase 225/100ug 125/100ug 1129 Antibody NF-kB inducing kinase 225/100ug 125/100ug 1131 Antibody TNFa converting enzyme 225/100ug 125/100ug 1133 Antibody GDNF receptor 225/100ug 125/100ug 1135 Antibody Neurturin receptor 225/100ug 125/100ug 1137 Antibody Persephin receptor 225/100ug 125/100ug 1139 Antibody Death Receptor for TRAIL 225/100ug 125/100ug 1141 Antibody DNA fragmentation factor & Inhibitor of CAD 225/100ug 125/100ug 1148 Antibody DNA fragmentation factor & Inhibitor of CAD 225/100ug 125/100ug 1150 Antibody Activator of MAPK pathway 225/100ug 125/100ug 1151 Antibody Apoptosis Signal-regulation Kinase 225/100ug 125/100ug 1156 Antibody FLICE inhibitory protein 225/100ug 125/100ug 1158 Antibody Cell Death Receptor 225/100ug 125/100ug 1159 -

TRPM7 Facilitates Cholinergic Vesicle Fusion with the Plasma Membrane
TRPM7 facilitates cholinergic vesicle fusion with the plasma membrane Sebastian Brauchi*, Grigory Krapivinsky, Luba Krapivinsky, and David E. Clapham† Department of Cardiology, Howard Hughes Medical Institute, Children’s Hospital Boston, and Department of Neurobiology, Harvard Medical School, Enders Building 1309, 320 Longwood Avenue, Boston, MA 02115 Contributed by David E. Clapham, January 29, 2008 (sent for review December 3, 2007) TRPM7, of the transient receptor potential (TRP) family, is both an the inclusion of a zinc-finger domain (13). This kinase phos- ion channel and a kinase. Previously, we showed that TRPM7 is phorylates TRPM7 (14) and other substrates [e.g., annexin 1 (15) located in the membranes of acetylcholine (ACh)-secreting synaptic and myosin heavy chain (16)], but its physiological role is not vesicles of sympathetic neurons, forms a molecular complex with known. The pore of TRPM7 was proposed to be a major entry proteins of the vesicular fusion machinery, and is critical for mechanism for Mg2ϩ ions and, in this role, critical for cell stimulated neurotransmitter release. Here, we targeted pHluorin survival (17). TRPM7 is localized in the membrane of sympa- to small synaptic-like vesicles (SSLV) in PC12 cells and demonstrate thetic neuronal acetylcholine (ACh)-secreting synaptic vesicles that it can serve as a single-vesicle plasma membrane fusion as part of a molecular complex of synaptic vesicle-specific reporter. In PC12 cells, as in sympathetic neurons, TRPM7 is located proteins and its permeability is critical -

Development and Validation of a Protein-Based Risk Score for Cardiovascular Outcomes Among Patients with Stable Coronary Heart Disease
Supplementary Online Content Ganz P, Heidecker B, Hveem K, et al. Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA. doi: 10.1001/jama.2016.5951 eTable 1. List of 1130 Proteins Measured by Somalogic’s Modified Aptamer-Based Proteomic Assay eTable 2. Coefficients for Weibull Recalibration Model Applied to 9-Protein Model eFigure 1. Median Protein Levels in Derivation and Validation Cohort eTable 3. Coefficients for the Recalibration Model Applied to Refit Framingham eFigure 2. Calibration Plots for the Refit Framingham Model eTable 4. List of 200 Proteins Associated With the Risk of MI, Stroke, Heart Failure, and Death eFigure 3. Hazard Ratios of Lasso Selected Proteins for Primary End Point of MI, Stroke, Heart Failure, and Death eFigure 4. 9-Protein Prognostic Model Hazard Ratios Adjusted for Framingham Variables eFigure 5. 9-Protein Risk Scores by Event Type This supplementary material has been provided by the authors to give readers additional information about their work. Downloaded From: https://jamanetwork.com/ on 10/02/2021 Supplemental Material Table of Contents 1 Study Design and Data Processing ......................................................................................................... 3 2 Table of 1130 Proteins Measured .......................................................................................................... 4 3 Variable Selection and Statistical Modeling ........................................................................................ -

Chemical Agent and Antibodies B-Raf Inhibitor RAF265
Supplemental Materials and Methods: Chemical agent and antibodies B-Raf inhibitor RAF265 [5-(2-(5-(trifluromethyl)-1H-imidazol-2-yl)pyridin-4-yloxy)-N-(4-trifluoromethyl)phenyl-1-methyl-1H-benzp{D, }imidazol-2- amine] was kindly provided by Novartis Pharma AG and dissolved in solvent ethanol:propylene glycol:2.5% tween-80 (percentage 6:23:71) for oral delivery to mice by gavage. Antibodies to phospho-ERK1/2 Thr202/Tyr204(4370), phosphoMEK1/2(2338 and 9121)), phospho-cyclin D1(3300), cyclin D1 (2978), PLK1 (4513) BIM (2933), BAX (2772), BCL2 (2876) were from Cell Signaling Technology. Additional antibodies for phospho-ERK1,2 detection for western blot were from Promega (V803A), and Santa Cruz (E-Y, SC7383). Total ERK antibody for western blot analysis was K-23 from Santa Cruz (SC-94). Ki67 antibody (ab833) was from ABCAM, Mcl1 antibody (559027) was from BD Biosciences, Factor VIII antibody was from Dako (A082), CD31 antibody was from Dianova, (DIA310), and Cot antibody was from Santa Cruz Biotechnology (sc-373677). For the cyclin D1 second antibody staining was with an Alexa Fluor 568 donkey anti-rabbit IgG (Invitrogen, A10042) (1:200 dilution). The pMEK1 fluorescence was developed using the Alexa Fluor 488 chicken anti-rabbit IgG second antibody (1:200 dilution).TUNEL staining kits were from Promega (G2350). Mouse Implant Studies: Biopsy tissues were delivered to research laboratory in ice-cold Dulbecco's Modified Eagle Medium (DMEM) buffer solution. As the tissue mass available from each biopsy was limited, we first passaged the biopsy tissue in Balb/c nu/Foxn1 athymic nude mice (6-8 weeks of age and weighing 22-25g, purchased from Harlan Sprague Dawley, USA) to increase the volume of tumor for further implantation. -

The TRPM7 Ion Channel Functions in Cholinergic Synaptic Vesicles and Affects Transmitter Release
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Neuron 52, 485–496, November 9, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.neuron.2006.09.033 The TRPM7 Ion Channel Functions in Cholinergic Synaptic Vesicles and Affects Transmitter Release Grigory Krapivinsky,1 Sumiko Mochida,3 (TRPML channels) are presumed intracellular vesicular Luba Krapivinsky,1 Susan M. Cibulsky,1 channels (Di Palma et al., 2002; LaPlante et al., 2002), and David E. Clapham1,2,* but their function in vesicles is not well understood. 1 Howard Hughes Medical Institute Neurotransmitter vesicles release their cargoes by Cardiology fusion with the plasma membrane followed by diffusion Children’s Hospital Boston of their contents into the intercellular space. The fusion 1309 Enders Building process itself is not completely understood, but involves 320 Longwood Avenue a complex of proteins with specialized functions that al- 2 Department of Neurobiology low them to achieve close approximation of the vesicle Harvard Medical School to the plasma membrane and sense changes in [Ca2+] Boston, Massachusetts 02115 that trigger rapid fusion (Sudhof, 2004). Although neuro- 3 Department of Physiology transmitter release requires ion channels, ion channels Tokyo Medical University are best known for their function as the plasma mem- Tokyo 160-8402 brane mediators of the timing and entry of Ca2+ into Japan the cell, which in turn triggers vesicle fusion. Functional ion channels have been recorded in preparations of syn- aptic vesicles (Yakir and Rahamimoff, 1995; Kelly and Summary Woodbury, 1996), and a few well-established plasma membrane channels have been proposed to localize to A longstanding hypothesis is that ion channels are vesicles and regulate their ionic equilibrium (Ehrenstein present in the membranes of synaptic vesicles and et al., 1991; Grahammer et al., 2001), but their molecular might affect neurotransmitter release. -

Free PDF Download
European Review for Medical and Pharmacological Sciences 2019; 23: 3088-3095 The effects of TRPM2, TRPM6, TRPM7 and TRPM8 gene expression in hepatic ischemia reperfusion injury T. BILECIK1, F. KARATEKE2, H. ELKAN3, H. GOKCE4 1Department of Surgery, Istinye University, Faculty of Medicine, VM Mersin Medical Park Hospital, Mersin, Turkey 2Department of Surgery, VM Mersin Medical Park Hospital, Mersin, Turkey 3Department of Surgery, Sanlıurfa Training and Research Hospital, Sanliurfa, Turkey 4Department of Pathology, Inonu University, Faculty of Medicine, Malatya, Turkey Abstract. – OBJECTIVE: Mammalian transient Introduction receptor potential melastatin (TRPM) channels are a form of calcium channels and they trans- Ischemia is the lack of oxygen and nutrients port calcium and magnesium ions. TRPM has and the cause of mechanical obstruction in sev- eight subclasses including TRPM1-8. TRPM2, 1 TRPM6, TRPM7, TRPM8 are expressed especial- eral tissues . Hepatic ischemia and reperfusion is ly in the liver cell. Therefore, we aim to investi- a serious complication and cause of cell death in gate the effects of TRPM2, TRPM6, TRPM7, and liver tissue2. The resulting ischemic liver tissue TRPM8 gene expression and histopathologic injury increases free intracellular calcium. Intra- changes after treatment of verapamil in the he- cellular calcium has been defined as an important patic ischemia-reperfusion rat model. secondary molecular messenger ion, suggesting MATERIALS AND METHODS: Animals were randomly assigned to one or the other of the calcium’s effective role in cell injury during isch- following groups including sham (n=8) group, emia-reperfusion, when elevated from normal verapamil (calcium entry blocker) (n=8) group, concentrations. The high calcium concentration I/R group (n=8) and I/R- verapamil (n=8) group.