Download The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
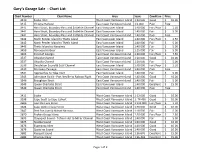
Gary's Charts
Gary’s Garage Sale - Chart List Chart Number Chart Name Area Scale Condition Price 3410 Sooke Inlet West Coast Vancouver Island 1:20 000 Good $ 10.00 3415 Victoria Harbour East Coast Vancouver Island 1:6 000 Poor Free 3441 Haro Strait, Boundary Pass and Sattelite Channel East Vancouver Island 1:40 000 Fair/Poor $ 2.50 3441 Haro Strait, Boundary Pass and Sattelite Channel East Vancouver Island 1:40 000 Fair $ 5.00 3441 Haro Strait, Boundary Pass and Sattelite Channel East Coast Vancouver Island 1:40 000 Poor Free 3442 North Pender Island to Thetis Island East Vancouver Island 1:40 000 Fair/Poor $ 2.50 3442 North Pender Island to Thetis Island East Vancouver Island 1:40 000 Fair $ 5.00 3443 Thetis Island to Nanaimo East Vancouver Island 1:40 000 Fair $ 5.00 3459 Nanoose Harbour East Vancouver Island 1:15 000 Fair $ 5.00 3463 Strait of Georgia East Coast Vancouver Island 1:40 000 Fair/Poor $ 7.50 3537 Okisollo Channel East Coast Vancouver Island 1:20 000 Good $ 10.00 3537 Okisollo Channel East Coast Vancouver Island 1:20 000 Fair $ 5.00 3538 Desolation Sound & Sutil Channel East Vancouver Island 1:40 000 Fair/Poor $ 2.50 3539 Discovery Passage East Coast Vancouver Island 1:40 000 Poor Free 3541 Approaches to Toba Inlet East Vancouver Island 1:40 000 Fair $ 5.00 3545 Johnstone Strait - Port Neville to Robson Bight East Coast Vancouver Island 1:40 000 Good $ 10.00 3546 Broughton Strait East Coast Vancouver Island 1:40 000 Fair $ 5.00 3549 Queen Charlotte Strait East Vancouver Island 1:40 000 Excellent $ 15.00 3549 Queen Charlotte Strait East -

Travel Green, Travel Locally Family Chartering
S WaS TERWaYS Natural History Coastal Adventures SPRING 2010 You select Travel Green, Travel Locally your adventure People travel across the world to experience different cultures, landscapes and learning. Yet, right here in North America we have ancient civilizations, But let nature untouched wilderness and wildlife like you never thought possible. Right here in our own backyard? select your Yes! It requires leaving the “highway” and taking a sense of exploration. But the reward is worth it, the highlights sense of adventure tangible. Bluewater explores coastal wilderness regions only The following moments accessible by boat. Our guided adventures can give await a lucky few… which you weeks worth of experiences in only 7-9 days. Randy Burke moments do you want? Learn about exotic creatures and fascinating art. Live Silently watching a female grizzly bear from kayaks in the your values and make your holidays green. Join us Great Bear Rainforest. • Witness bubble-net feeding whales in (and find out what all the fuss is about). It is Southeast Alaska simple… just contact us for available trip dates and Bluewater Adventures is proud to present small group, • Spend a quiet moment book your Bluewater Adventure. We are looking carbon neutral trips for people looking for a different in SGang Gwaay with forward to seeing you at that small local airport… type of “cruise” since 1974. the ancient spirits and totems • See a white Spirit bear in the Great Bear Family Chartering Rainforest “Once upon a time… in late July of 2009, 13 experiences of the trip and • Stand inside a coastal members of a very diverse and far flung family flew savoring our family. -

Oceans, Habitat and Enhancement Branch 2006-2007
Oceans, Habitat and Enhancement Branch 2006-2007 DirectoryA guide to community involvement, stewardship, Streamkeepers, and education projects in British Columbia and the Yukon Territory Published by Community Involvement Oceans, Habitat and Enhancement Branch Fisheries and Oceans Canada Suite 200 – 401 Burrard Street Vancouver, BC V6C 3S4 Dear Stewardship Community, This edition of the Stewardship and Community Involvement directory marks our 15th year of publication. We believe this is a useful reference tool, providing a summary of the numerous community-based projects and activities that partner with Oceans, Habitat and Enhancement Community Programs. This edition is organized by geographic areas to reflect the area-based management model which Fisheries and Oceans Canada has implemented in the Pacific Region. The future of our world depends upon educating children and young adults. The Stream to Sea education program is strongly supported throughout Pacific Region, with involvement of over 25 part and full-time Education Coordinators, 18 Community Advisors and many educational professionals and volunteers supporting the program. The Stream to Sea program combines oceans and aquatic species education and lessons on marine and freshwater habitat to create a stewardship ethic. The ultimate goal is to have students become aquatic stewards, caring for the environment around them. The Community Advisors dedicate their mission statement to the volunteers and community projects: “Fostering cooperative fisheries and watershed stewardship through education and involvement”. Our Community Advisors work alongside the stewardship community, building partnerships within community. From assisting with mini hatchery programs, policy implementation, to taking an active role in oceans and watershed planning, these staff members are the public face of DFO. -

DISCOVERY PASSAGE SCHOOL CLOSU RE CONSULTATION PROCESS Late Submissions
DISCOVERY PASSAGE SCHOOL CLOSU RE CONSULTATION PROCESS Late Submissions CONSULTATION INDEX DATE DESCRIPTION 03-05-2016 Claire Metcalfe 03-01-2016 Curtis and Amanda Smith i Lee-Ann Kruse From: Claire Metcalfe <[email protected]> Sent: March-OS-16 9:09 AM To: facilities plan; Susan Wilson; Ted Foster; Richard Franklin; Daryl Hagen; John Kerr; Gail Kirschner; Joyce McMann Cc: [email protected]; [email protected]; [email protected] Subject Fwd: SD72 School Closures Trolly kids All of the students in our district are standing on the train tracks and there is an out of control trolly headed for them all. Do we toss a few in the way (the elementary school kids who will also be tossed again when it comes to rebuilding the high school) to prevent them from all being hurt? Or do we ask no one to budge and see who survives? This is a morality question that has been presented to the trustees before, and I believe at a very appropriate time. Lers do something different. Pick an answer that isn't already proposed. I propose that we ask them to all step away from the tracks and let the train (Christy Clark) go along on it's merry way. I know the solution is not that simple. There has to be another way of building new schools in our district and supporting our childrens education other than following the paths that other districts are, just because it is what we are supposed to. Our district staff and trustees are smart, creative leaders of our community, and I would like for them to come up with another way to go about this. -

Gaytan to Marin Donald Cutter the Spanish Legend, That
The Spanish in Hawaii: Gaytan to Marin Donald Cutter The Spanish legend, that somehow Spain anticipated all other Europeans in its discovery and presence in most every part of the New World, extends even to the Pacific Ocean area. Spain's early activity in Alaska, Canada, Washington, Oregon, and California reinforces the idea that Spain was also the early explorer of the Pacific Islands. The vast Pacific, from its European discovery in Panama by Vasco Nunez de Balboa, until almost the end of the 18th Century, was part of the Spanish overseas empire. Generous Papal recognition of Spain's early discoveries and an attempt to avert an open conflict between Spain and Portugal resulted in a division of the non-Christian world between those Iberian powers. Though north European nations were not in accord and the King of France even suggested that he would like to see the clause in Adam's will giving the Pope such sweeping jurisdiction, Spain was convinced of its exclusive sovereignty over the Pacific Ocean all the way to the Philippine Islands. Spain strengthened both the Papal decree and the treaty signed with Portugal at Tordasillas by observing the niceties of international law. In 1513, Nunez de Balboa waded into the Pacific, banner in hand, and in a single grandiose act of sovereignty claimed the ocean and all of its islands for Spain. It was a majestic moment in time—nearly one third of the world was staked out for exclusive Spanish control by this single imperial act. And Spain was able to parlay this act of sovereignty into the creation of a huge Spanish lake of hundreds of thousands of square miles, a body of water in which no other European nation could sail in peaceful commerce. -

BUILDING the FUTURE KELOWNA Aboriginal Training and Mentoring Farmers’ Delights
In-flight Magazine for Pacific Coastal Airlines BOOMING Vancouver Island construction on the rise TASTY BUILDING THE FUTURE KELOWNA Aboriginal training and mentoring Farmers’ delights June /July 2014 | Volume 8 | Number 3 NEW PRICE ED HANDJA Personal Real Estate Corporation & SHELLEY MCKAY Your BC Oceanfront Team Specializing in Unique Coastal Real Estate in British Columbia Ed 250.287.0011 • Shelley 250.830.4435 Toll Free 800.563.7322 [email protected] [email protected] Great Choices for Recreational Use & Year-round Living • www.bcoceanfront.com • Great Choices for Recreational Use & Year-round Living • www.bcoceanfront.com Use & Year-round • Great Choices for Recreational Living • www.bcoceanfront.com Use & Year-round Great Choices for Recreational West Coast Vancouver Island: Three 10 acre Kyuquot Sound, Walters Cove: Premier shing Sonora Island Oceanfront: This one has it all - oceanfront properties next to the Broken Island and outdoor recreation from this west coast 3 acre property with 400ft low-bank oceanfront, Marine Group. 275ft – 555ft of low bank beach Vancouver Island community. Government dock good, protected moorage, 4 dwellings, gardens, a front. There are roughed in internal access trails and general store, power and water. beautiful setting and wonderful views. Sheltered and a shared rock jetty for of oading. Water 1100sqft classic home, new private moorage location, southern exposure, water licenses access only properties. Region renowned for $224,900 for domestic water and power generation. An shing, whale watching and boating. Great value. Older homestead, private moorage $184,900 ideal remote residence or lodge in the popular $83,600 - $103,400 1000sqft 2bdrm home, plus full basement $199,000 Discovery Islands. -

British Columbia Regional Guide Cat
National Marine Weather Guide British Columbia Regional Guide Cat. No. En56-240/3-2015E-PDF 978-1-100-25953-6 Terms of Usage Information contained in this publication or product may be reproduced, in part or in whole, and by any means, for personal or public non-commercial purposes, without charge or further permission, unless otherwise specified. You are asked to: • Exercise due diligence in ensuring the accuracy of the materials reproduced; • Indicate both the complete title of the materials reproduced, as well as the author organization; and • Indicate that the reproduction is a copy of an official work that is published by the Government of Canada and that the reproduction has not been produced in affiliation with or with the endorsement of the Government of Canada. Commercial reproduction and distribution is prohibited except with written permission from the author. For more information, please contact Environment Canada’s Inquiry Centre at 1-800-668-6767 (in Canada only) or 819-997-2800 or email to [email protected]. Disclaimer: Her Majesty is not responsible for the accuracy or completeness of the information contained in the reproduced material. Her Majesty shall at all times be indemnified and held harmless against any and all claims whatsoever arising out of negligence or other fault in the use of the information contained in this publication or product. Photo credits Cover Left: Chris Gibbons Cover Center: Chris Gibbons Cover Right: Ed Goski Page I: Ed Goski Page II: top left - Chris Gibbons, top right - Matt MacDonald, bottom - André Besson Page VI: Chris Gibbons Page 1: Chris Gibbons Page 5: Lisa West Page 8: Matt MacDonald Page 13: André Besson Page 15: Chris Gibbons Page 42: Lisa West Page 49: Chris Gibbons Page 119: Lisa West Page 138: Matt MacDonald Page 142: Matt MacDonald Acknowledgments Without the works of Owen Lange, this chapter would not have been possible. -

Assessing the Site Potential for Underwater Turbines in Tidal Channels Using Numerical Modeling and Advanced Ocean Current Measurements
Assessing the Site Potential for Underwater Turbines in Tidal Channels Using Numerical Modeling and Advanced Ocean Current Measurements David B. Fissel, Jianhua Jiang, Rick Birch, Jan Buermans and David Lemon ASL Environmental Sciences Inc., 1986 Mills Rd., Sidney, BC, V8L 5Y3, Canada, Phone Number (250) 656-0177, [email protected] Abstract -A combination of advanced ocean current profiling level differences exist between two sides of the dam with the measurements and high resolution 3D numerical models was maximum heads up to 1.5 m during spring tides and 0.8 m used to assess site potential for underwater turbines in tidal during neap tides. The difference in water levels on either channels of the inland waters off the coast of British Columbia, side of the dam has the potential for significant renewable Canada. The measurements involved the use of ADCP transects energy through installation of underwater turbines for through potential sites. Due to the very strong tidal currents of up to 10 knots or more, special procedures are required to generating electrical power. Numerical modeling simulations generate accurate and reliable maps of the very strong ocean of the currents and water levels were conducted, including the currents. The three-dimensional, coastal circulation model present conditions for model calibration and verification, COCIRM was used to map these detailed flows under different possible future conditions in which the dam is completely scenarios and assess the potential at various sites for operation removed and the Pass is restored to its original configuration, of underwater turbines after validated using available water and possible future conditions in which the dam is partially elevation and ocean current data. -

Copyright (C) Queen's Printer, Victoria, British Columbia, Canada
B.C. Reg. 38/2016 O.C. 112/2016 Deposited February 29, 2016 effective February 29, 2016 Water Sustainability Act WATER DISTRICTS REGULATION Note: Check the Cumulative Regulation Bulletin 2015 and 2016 for any non-consolidated amendments to this regulation that may be in effect. Water districts 1 British Columbia is divided into the water districts named and described in the Schedule. Schedule Water Districts Alberni Water District That part of Vancouver Island together with adjacent islands lying southwest of a line commencing at the northwest corner of Fractional Township 42, Rupert Land District, being a point on the natural boundary of Fisherman Bay; thence in a general southeasterly direction along the southwesterly boundaries of the watersheds of Dakota Creek, Laura Creek, Stranby River, Nahwitti River, Quatse River, Keogh River, Cluxewe River and Nimpkish River to the southeasterly boundary of the watershed of Nimpkish River; thence in a general northeasterly direction along the southeasterly boundary of the watershed of Nimpkish River to the southerly boundary of the watershed of Salmon River; thence in a general easterly direction along the southerly boundary of the watershed of Salmon River to the southwesterly boundary thereof; thence in a general southeasterly direction along the southwesterly boundaries of the watersheds of Salmon River and Campbell River to the southerly boundary of the watershed of Campbell River; thence in a general easterly direction along the southerly boundaries of the watersheds of Campbell River and -

Original Field Data and Traverse Notes Must Be Provided by the Licensee
Ministry of Forests, Lands, Natural Resource Operations and Rural Development Minister’s Office MEMORANDUM Cliff: 259440 Ref: 280-20 November 25, 2020 To: Sharon Hadway, Regional Executive Director, West Coast Allan Johnsrude, Regional Executive Director, South Coast From: The Honourable Doug Donaldson Minister of Forests, Lands, Natural Resource Operations and Rural Development Re: New Coast Appraisal Manual I hereby approve the new Coast Appraisal Manual and attach a copy for your use. The manual is available at the following link: https://www2.gov.bc.ca/gov/content/industry/forestry/competitive-forest-industry/ timber-pricing/coast-timber-pricing/coast-appraisal-manual-and-amendments This manual will come into force on December 15, 2020. Further amendments or revisions to this manual require my approval. Minister pc: Melissa Sanderson, Assistant Deputy Minister, Forest Policy and Indigenous Relations Division Jim Schafthuizen, Executive Director, Forest Policy and Indigenous Relations Division Allan Bennett, Director, Timber Pricing Branch TIMBER PRICING BRANCH Coast Appraisal Manual Effective December 15, 2020 This manual is intended for the use of individuals or companies when conducting business with the British Columbia Government. Permission is granted to reproduce it for such purposes. This manual and related documentation and publications, are protected under the Federal Copyright Act. They may not be reproduced for sale or for other purposes without the express written permission of the Province of British Columbia. Coast Appraisal Manual Highlights New Coast Appraisal Manual Highlights The new Coast Appraisal Manual includes clarification to policy, an update to the market pricing system, and an update of the tenure obligation adjustments and specified operations for December 15, 2020 onward. -

Western Canada Explorer Featuring Vancouver, Victoria and Whistler
Antioch Seniors AND TravelCenter Travel & Tours presents... 9 DAY HOLIDAY Western Canada Explorer featuring Vancouver, Victoria and Whistler July 24 - August 1, 2020 Tour Dates: Western Canada Explorer Unforgettable experiences await 9 Days • 15 Meals in Canada’s Golden Triangle featuring mountain gondolas, a First Nations cultural experience, a regional Foodie Tour and an incredible wildlife cruise. TOUR HIGHLIGHTS 4 15 Meals (8 breakfasts, 3 lunches and 4 dinners) 4 Round trip airport transfers 4 Spend 3 nights in cosmopolitan Vancouver 4 Take a panoramic tour of Vancouver to see its downtown core, spectacular North Shore and beautiful Stanley Park and visit Capilano Suspension Bridge 4 Travel the scenic “Sea to Sky Highway” to and enjoy the PEAK 2 PEAK experience, a 1.88-mile long gondola ride between Blackcomb and Whistler Mountains 4 Travel by BC Ferry to Vancouver Island and visit world-famous Butchart Gardens 4 Included city tour of Victoria with its delightful English flavor, red double-decker buses and Tudor-style buildings Cross the Capilano Suspension Bridge and enjoy views of the spectacular rainforest 4 Visit Victorian-era Craigdarroch Castle and take the walking Victoria Food Tour, a delicious culinary experience 4 Enjoy a First Nations Cultural Experience at the I-Hos Gallery DAY 1 – Arrive in Beautiful British Columbia featuring a weaving workshop and included lunch with traditional Welcome to Canada’s rugged Northwest in Vancouver and transfer Bannock bread to your hotel. Meet your Tour Manager in the hotel lobby at 6:00 4 Spend 2 nights at the illustrious Painter’s Lodge, located on the p.m. -

RG 42 - Marine Branch
FINDING AID: 42-21 RECORD GROUP: RG 42 - Marine Branch SERIES: C-3 - Register of Wrecks and Casualties, Inland Waters DESCRIPTION: The finding aid is an incomplete list of Statement of Shipping Casualties Resulting in Total Loss. DATE: April 1998 LIST OF SHIPPING CASUALTIES RESULTING IN TOTAL LOSS IN BRITISH COLUMBIA COASTAL WATERS SINCE 1897 Port of Net Date Name of vessel Registry Register Nature of casualty O.N. Tonnage Place of casualty 18 9 7 Dec. - NAKUSP New Westminster, 831,83 Fire, B.C. Arrow Lake, B.C. 18 9 8 June ISKOOT Victoria, B.C. 356 Stranded, near Alaska July 1 MARQUIS OF DUFFERIN Vancouver, B.C. 629 Went to pieces while being towed, 4 miles off Carmanah Point, Vancouver Island, B.C. Sept.16 BARBARA BOSCOWITZ Victoria, B.C. 239 Stranded, Browning Island, Kitkatlah Inlet, B.C. Sept.27 PIONEER Victoria, B.C. 66 Missing, North Pacific Nov. 29 CITY OF AINSWORTH New Westminster, 193 Sprung a leak, B.C. Kootenay Lake, B.C. Nov. 29 STIRINE CHIEF Vancouver, B.C. Vessel parted her chains while being towed, Alaskan waters, North Pacific 18 9 9 Feb. 1 GREENWOOD Victoria, B.C. 89,77 Fire, laid up July 12 LOUISE Seaback, Wash. 167 Fire, Victoria Harbour, B.C. July 12 KATHLEEN Victoria, B.C. 590 Fire, Victoria Harbour, B.C. Sept.10 BON ACCORD New Westminster, 52 Fire, lying at wharf, B.C. New Westminster, B.C. Sept.10 GLADYS New Westminster, 211 Fire, lying at wharf, B.C. New Westminster, B.C. Sept.10 EDGAR New Westminster, 114 Fire, lying at wharf, B.C.