1 FLAMINGO 2019 EARLY VIEW New Nesting Sites of the Threatened
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

6-A John James Audubon, American Flamingo, 1838
JOHN JAMES AUDUBON [1785–1851] 6 a American Flamingo,1838 American Flamingo is one of the 435 hand-colored engravings that River, a major flyway for migratory birds, and eventually wan- make up John James Audubon’s monumental Birds of America, dered farther from home to comb the American frontier for issued in four volumes between 1826 and 1838. The massive unrecorded species. publication includes life-size representations of nearly five hundred Audubon’s procedure was to study and sketch a bird in its natural species of North American birds. Although Audubon was not the habitat before killing it carefully, using fine shot to minimize dam- first to attempt such a comprehensive catalog, his work departed age. His critical innovation was to then thread wire through the from conventional scientific illustration, which showed lifeless spec- specimen, allowing him to fashion a lifelike pose. He worked in imens against a blank background, by presenting the birds as they watercolor, and had completed some four hundred paintings appeared in the wild. When his pictures were first published, when he decided to publish them as a folio of prints. Failing to find some naturalists objected to Audubon’s use of dramatic action and support in Philadelphia, he sailed for England, where he became pictorial design, but these are the qualities that set his work apart lionized as “The American Woodsman.” The engraving firm and make it not only an invaluable record of early American Robert Havell and Son took on the challenge of reproducing wildlife but an unmatched work of American art. Audubon’s paintings on copper plates and tinting the resulting John James Audubon was born in Haiti and educated in France, black-and-white prints by hand. -

A Pliocene Flamingo from Mexico
June, 1944 THE WILSON BULLETIN 77 Vol. 56. No. 2 A PLIOCENE FLAMINGO FROM MEXICO BY LOYE MILLER IELD parties from the California Institute of Technology have been F fortunate in locating a variety of fossil deposits in Mexico that in- cluded bird remains. Some have been very rich in the quantity and variety of material; for example, the San Josecito Cavern of Nuevo Leon (Miller, ,1943), a deposit of Pleistocene age, yielded several thousand bird bones assigned to over forty species. The present paper deals with a collection of ten fragments, all but one of which are in- cluded in a single species. I am indebted to Dr. Chester Stock in charge of the explorations for the opportunity of working with the bird collec- tions. Dr. Alexander Wetmore has loaned comparative material, and Dr. Hildegarde Howard has been a most congenial fellow student during many conferences on the flamingoes, both Recent and Fossil. To these several colleagues my sincere thanks are offered. The ten fragments are from collecting locality No. 289, California Institute of Technology, known. as the Rincon Pliocene, Chihuahua, Mexico. Associated mammal remains include horse, camel, antelope, and carnivore species. The matrix is a fine grained silt of lightest color, without cementing material. A stiff brush serves to remove it from the well petrified bones. Unfortunately the specimens are most frag- mentary. They do, however, prove to be of interest in several respects; most notably they prove (since several speciments are from pre-volant young) that a small speciesof flamingo was present as a breeding bird. This is the earliest record for the family in America. -

Flamingo ABOUT the GROUP
Flamingo ABOUT THE GROUP Bulletin of the IUCN-SSC/Wetlands International The Flamingo Specialist Group (FSG) was established in 1978 at Tour du Valat in France, under the leadership of Dr. Alan Johnson, who coordinated the group until 2004 (see profile at www.wetlands.org/networks/Profiles/January.htm). Currently, the group is FLAMINGO SPECIALIST GROUP coordinated from the Wildfowl & Wetlands Trust at Slimbridge, UK, as part of the IUCN- SSC/Wetlands International Waterbird Network. The FSG is a global network of flamingo specialists (both scientists and non- scientists) concerned with the study, monitoring, management and conservation of the world’s six flamingo species populations. Its role is to actively promote flamingo research and conservation worldwide by encouraging information exchange and cooperation amongst these specialists, and with other relevant organisations, particularly IUCN - SSC, Ramsar, WWF International and BirdLife International. FSG members include experts in both in-situ (wild) and ex-situ (captive) flamingo conservation, as well as in fields ranging from field surveys to breeding biology, diseases, tracking movements and data management. There are currently 165 members around the world, from India to Chile, and from France to South Africa. Further information about the FSG, its membership, the membership list serve, or this bulletin can be obtained from Brooks Childress at the address below. Chair Assistant Chair Dr. Brooks Childress Mr. Nigel Jarrett Wildfowl & Wetlands Trust Wildfowl & Wetlands Trust Slimbridge Slimbridge Glos. GL2 7BT, UK Glos. GL2 7BT, UK Tel: +44 (0)1453 860437 Tel: +44 (0)1453 891177 Fax: +44 (0)1453 860437 Fax: +44 (0)1453 890827 [email protected] [email protected] Eastern Hemisphere Chair Western Hemisphere Chair Dr. -
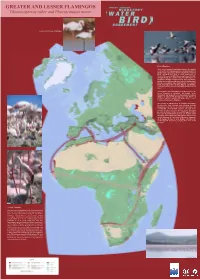
GREATER and LESSER FLAMINGOS Phoenicopterus Ruber and Phoeniconaias Minor
GREATER AND LESSER FLAMINGOS Phoenicopterus ruber and Phoeniconaias minor Greater and Lesser Flamingos © Cliff Buckton © P & H Harris Lesser Flamingo The Lesser Flamingo Phoeniconaias minor is the smallest of the world's five flamingo species. It occurs primarily in the Rift Valley lakes of East Africa with about 4 to 5 million birds estimated, but also in small populations in Namibia/Botswana (40,000), Mauritania/Senegal (15,400), Ethiopia (8,300). The alkaline lakes of the Rift Valley are the primary feeding areas for the East Africa population. During non-breeding periods these lakes often hold almost the entire population. Huge feeding flocks of 1-2 million birds frequently gather on lakes Bogoria and Nakuru, creating one of the most stunning wildlife spectacles in the world. Although it is still the most numerous of the five species, the Lesser Flamingo is classified as globally "near threatened" due primarily to its dependence on a limited number of unprotected breeding sites and threats of proposed soda-ash mining and hydro-electric power schemes on the main breeding lakes. The question of whether there is occasional interchange between the East African and southern African populations has yet to be resolved definitely, but considerable circumstantial evidence has now been assembled to show that East African Lesser Flamingos probably do fly to Botswana to breed during periods when the Lake Makgadikgadi Salt Pans are flooded. Their migration routes, flight range and stopover places (if any) are still unknown. It is now known that Lesser Flamingos do fly during the day, at great heights, well above the normal diurnal movement of eagles, their main aerial predator. -

Flamingo Newsletter 17, 2009
ABOUT THE GROUP The Flamingo Specialist Group (FSG) is a global network of flamingo specialists (both scientists and non-scientists) concerned with the study, monitoring, management and conservation of the world’s six flamingo species populations. Its role is to actively promote flamingo research, conservation and education worldwide by encouraging information exchange and cooperation among these specialists, and with other relevant organisations, particularly the IUCN Species Survival Commission (SSC), the Ramsar Convention on Wetlands, the Convention on Conservation of Migratory Species (CMS), the African-Eurasian Migratory Waterbird Agreement (AEWA), and BirdLife International. The group is coordinated from the Wildfowl & Wetlands Trust, Slimbridge, UK, as part of the IUCN-SSC/Wetlands International Waterbird Network. FSG members include experts in both in-situ (wild) and ex-situ (captive) flamingo conservation, as well as in fields ranging from research surveys to breeding biology, infectious diseases, toxicology, movement tracking and data management. There are currently 286 members representing 206 organisations around the world, from India to Chile, and from France to South Africa. Further information about the FSG, its membership, the membership list serve, or this bulletin can be obtained from Brooks Childress at the address below. Chair Dr. Brooks Childress Wildfowl & Wetlands Trust Slimbridge Glos. GL2 7BT, UK Tel: +44 (0)1453 860437 Fax: +44 (0)1453 860437 [email protected] Eastern Hemisphere Chair Western Hemisphere Chair Dr. Arnaud Béchet Dr. Felicity Arengo Station biologique, Tour du Valat American Museum of Natural History Le Sambuc Central Park West at 79th Street 13200 Arles, France New York, NY 10024 USA Tel : +33 (0) 4 90 97 20 13 Tel: +1 212 313-7076 Fax : +33 (0) 4 90 97 20 19 Fax: +1 212 769-5292 [email protected] [email protected] Citation: Childress, B., Arengo, F. -

1 ID Euring Latin Binomial English Name Phenology Galliformes
BIRDS OF METAURO RIVER: A GREAT ORNITHOLOGICAL DIVERSITY IN A SMALL ITALIAN URBANIZING BIOTOPE, REQUIRING GREATER PROTECTION 1 SUPPORTING INFORMATION / APPENDICE Check list of the birds of Metauro river (mouth and lower course / Fano, PU), up to September 2020. Lista completa delle specie ornitiche del fiume Metauro (foce e basso corso /Fano, PU), aggiornata ad Settembre 2020. (*) In the study area 1 breeding attempt know in 1985, but in particolar conditions (Pandolfi & Giacchini, 1985; Poggiani & Dionisi, 1988a, 1988b, 2019). ID Euring Latin binomial English name Phenology GALLIFORMES Phasianidae 1 03700 Coturnix coturnix Common Quail Mr, B 2 03940 Phasianus colchicus Common Pheasant SB (R) ANSERIFORMES Anatidae 3 01690 Branta ruficollis The Red-breasted Goose A-1 (2012) 4 01610 Anser anser Greylag Goose Mi, Wi 5 01570 Anser fabalis Tundra/Taiga Bean Goose Mi, Wi 6 01590 Anser albifrons Greater White-fronted Goose A – 4 (1986, february and march 2012, 2017) 7 01520 Cygnus olor Mute Swan Mi 8 01540 Cygnus cygnus Whooper Swan A-1 (1984) 9 01730 Tadorna tadorna Common Shelduck Mr, Wi 10 01910 Spatula querquedula Garganey Mr (*) 11 01940 Spatula clypeata Northern Shoveler Mr, Wi 12 01820 Mareca strepera Gadwall Mr, Wi 13 01790 Mareca penelope Eurasian Wigeon Mr, Wi 14 01860 Anas platyrhynchos Mallard SB, Mr, W (R) 15 01890 Anas acuta Northern Pintail Mi, Wi 16 01840 Anas crecca Eurasian Teal Mr, W 17 01960 Netta rufina Red-crested Pochard A-4 (1977, 1994, 1996, 1997) 18 01980 Aythya ferina Common Pochard Mr, W 19 02020 Aythya nyroca Ferruginous -

The Handrearing of a Chilian Flamingo And
£; 'E (j) CJ) :§ o > .0 o (5 .c 0.. PInk Floyd The Handrearing ofa Chilian Flamingo and its Introduction to the Flock Floyd (second from right) seen as a member ofthe flock. He can be distinguished be the light by Chris Smith gray down on the headand neckand by the lighter shade ofpink on the body andwings. Shortly Animal Technician after Floyd was introduced to the flock, several pairs C!f.flamingos successfully hatched and Oklahoma City Zoological Park reared theiryoung. ink Floyd: to many people it is birds care. However, after one of the hatching, chicks stay in or near the the name of a rock band that has first eggs disappeared from the nest, nest for about the first week. After Precorded many cIa sic albums the remaining and subsequent eggs leaving the nest, chicks congregate such as "The Wall" and "The Dark Side were pulled and replaced with dummy with other chicks, forming creches or of the Moon. ' To the personnel at the eggs. The eggs were placed in incuba nursery groups. These creches can Oklahoma City Zoological Park, it is also tor at a temperature of 99.5° F. (37.5° consist of several thousand young the name of its first captive-hatched C). The hygrometer was maintained birds. Chilean Flamingo, Phoenicopterus between 82-84°F (30-31°C). The eggs Adult flamingos take turns watching chilensis. were turned five times daily. A total of over the chicks. While the chicks are in The Oklahoma City Zoo has exhib eight flamingo eggs were pulled from the nursery, the parents continue to ited Chilean Flamingos since 1966, but the nests. -

Modern Birds Classification System Tinamiformes
6.1.2011 Classification system • Subclass: Neornites (modern birds) – Superorder: Paleognathae, Neognathae Modern Birds • Paleognathae – two orders, 49 species • Struthioniformes—ostriches, emus, kiwis, and allies • Tinamiformes—tinamous Ing. Jakub Hlava Department of Zoology and Fisheries CULS Tinamiformes • flightless • Dwarf Tinamou • consists of about 47 species in 9 genera • Dwarf Tinamou ‐ 43 g (1.5 oz) and 20 cm (7.9 in) • Gray Tinamou ‐ 2.3 kg (5.1 lb) 53 cm (21 in) • small fruits and seeds, leaves, larvae, worms, and mollusks • Gray Tinamou 1 6.1.2011 Struthioniformes Struthioniformes • large, flightless birds • Ostrich • most of them now extinct • Cassowary • chicks • Emu • adults more omnivorous or insectivorous • • adults are primarily vegetarian (digestive tracts) Kiwi • Emus have a more omnivorous diet, including insects and other small animals • kiwis eat earthworms, insects, and other similar creatures Neognathae Galloanserae • comprises 27 orders • Anseriformes ‐ waterfowl (150) • 10,000 species • Galliformes ‐ wildfowl/landfowl (250+) • Superorder Galloanserae (fowl) • Superorder Neoaves (higher neognaths) 2 6.1.2011 Anseriformes (screamers) Anatidae (dablling ducks) • includes ducks, geese and swans • South America • cosmopolitan distribution • Small group • domestication • Large, bulky • hunted animals‐ food and recreation • Small head, large feet • biggest genus (40‐50sp.) ‐ Anas Anas shoveler • mallards (wild ducks) • pintails • shlhovelers • wigeons • teals northern pintail wigeon male (Eurasian) 3 6.1.2011 Tadorninae‐ -

Ward-Nutrient Composition of American Flamingo Crop Milk.Pdf
Nutrient Composition Of American Flamingo Crop Milk Ann M. Ward1, Amy Hunt1, Mike Maslanka1*, and Chris Brown2 1 Nutritional Services Department, Fort Worth Zoo, Fort Worth, Texas USA 2Bird Department, Dallas Zoo, Dallas, Texas USA Crop milk samples (30 mL) were collected from juvenile (6-7 wks old) American flamingos (Phoenicopterus ruber ruber, n = 14) in the Ria Lagartos Biosphere Reserve (El Cuyo, Mexico) on the northern coast of the Yucatan Peninsula. The samples were analyzed for dry matter, crude protein, fat, minerals (calcium, phosphorus, magnesium, manganese, sodium, potassium, iron, copper, manganese, molybdenum, zinc), vitamin A, vitamin E, lutein and zeaxanthin, beta-cryptoxanthin, echinenone, canthaxanthin, and beta-carotene. In addition, morphometric measurements and blood samples were taken at the time of sample collection. This information will allow us to learn more about crop milk and avian lactation, and, most practically, to aid in flamingo hand-rearing efforts. Key words: Phoenicopterus ruber ruber, carotenoids, holocrine secretion INTRODUCTION American flamingos (Phoenicopterus ruber ruber) occur throughout the coastal wetland system of the Yucatan Peninsula. One of the largest flamingo colonies nests in the Ria Lagartos Lagoon on the northeastern coast. These birds utilize the surrounding habitat, which includes local commercial salt production operations, for roosting and foraging throughout the year [Arengo and Baldassarre, 1998]. Flamingos support their young for up to six months with crop milk. Along with several species of pigeons and doves, and some penguins, flamingos share with mammals the ability to secrete milk for the nourishment of their young. Much like in mammals, the production of milk in birds is prolactin mediated. -

Evidence for Aviculture: Identifying Research Needs to Advance the Role of Ex Situ Bird Populations in Conservation Initiatives and Collection Planning
Review Evidence for Aviculture: Identifying Research Needs to Advance the Role of Ex Situ Bird Populations in Conservation Initiatives and Collection Planning Paul Rose 1,2 1 Centre for Research in Animal Behaviour, Psychology, University of Exeter, Perry Road, Exeter, Devon EX4 4QG, UK; [email protected] or [email protected] 2 WWT, Slimbridge Wetland Centre, Slimbridge, Gloucestershire GL2 7BT, UK Simple Summary: Birds of a whole range of species are housed in zoological collections globally; they are some of the most frequently seen of species in animal populations kept under human care. Research output on birds can provide valuable information on how to advance husbandry and care for particular species, which may further feed into conservation planning. Linking birds housed in human care to those in the wild adds value to these zoo-housed populations; this paper provides areas of research that could be conducted to add value to these zoo-housed birds and suggests increasing the conservation focus and conservation relevance of birds housed by humans. Abstract: Birds are the most speciose of all taxonomic groups currently housed in zoos, but this species diversity is not always matched by their inclusion in research output in the peer-reviewed literature. This large and diverse captive population is an excellent tool for research investigation, the findings of which can be relevant to conservation and population sustainability aims. The One Plan Approach to conservation aims to foster tangible conservation relevance of ex situ populations to those animals living in situ. The use of birds in zoo aviculture as proxies for wild-dwelling Citation: Rose, P. -

Between Species: Choreographing Human And
BETWEEN SPECIES: CHOREOGRAPHING HUMAN AND NONHUMAN BODIES JONATHAN OSBORN A DISSERTATION SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY GRADUATE PROGRAM IN DANCE STUDIES YORK UNIVERSITY TORONTO, ONTARIO MAY, 2019 ã Jonathan Osborn, 2019 Abstract BETWEEN SPECIES: CHOREOGRAPHING HUMAN AND NONHUMAN BODIES is a dissertation project informed by practice-led and practice-based modes of engagement, which approaches the space of the zoo as a multispecies, choreographic, affective assemblage. Drawing from critical scholarship in dance literature, zoo studies, human-animal studies, posthuman philosophy, and experiential/somatic field studies, this work utilizes choreographic engagement, with the topography and inhabitants of the Toronto Zoo and the Berlin Zoologischer Garten, to investigate the potential for kinaesthetic exchanges between human and nonhuman subjects. In tracing these exchanges, BETWEEN SPECIES documents the creation of the zoomorphic choreographic works ARK and ARCHE and creatively mediates on: more-than-human choreography; the curatorial paradigms, embodied practices, and forms of zoological gardens; the staging of human and nonhuman bodies and bodies of knowledge; the resonances and dissonances between ethological research and dance ethnography; and, the anthropocentric constitution of the field of dance studies. ii Dedication Dedicated to the glowing memory of my nana, Patricia Maltby, who, through her relentless love and fervent belief in my potential, elegantly willed me into another phase of life, while she passed, with dignity and calm, into another realm of existence. iii Acknowledgements I would like to thank my phenomenal supervisor Dr. Barbara Sellers-Young and my amazing committee members Dr. -

AMNH-Scientific-Publications-2014
AMERICAN MUSEUM OF NATURAL HISTORY Fiscal Year 2014 Scientific Publications Division of Anthropology 2 Division of Invertebrate Zoology 11 Division of Paleontology 28 Division of Physical Sciences 39 Department of Earth and Planetary Sciences and Department of Astrophysics Division of Vertebrate Zoology Department of Herpetology 58 Department of Ichthyology 62 Department of Mammalogy 65 Department of Ornithology 78 Center for Biodiversity and Conservation 91 Sackler Institute for Comparative Genomics 99 DIVISION OF ANTHROPOLOGY Berwick, R.C., M.D. Hauser, and I. Tattersall. 2013. Neanderthal language? Just-so stories take center stage. Frontiers in Psychology 4, article 671. Blair, E.H., and Thomas, D.H. 2014. The Guale uprising of 1597: an archaeological perspective from Mission Santa Catalina de Guale (Georgia). In L.M. Panich and T.D. Schneider (editors), Indigenous Landscapes and Spanish Missions: New Perspectives from Archaeology and Ethnohistory: 25–40. Tucson: University of Arizona Press. Charpentier, V., A.J. de Voogt, R. Crassard, J.-F. Berger, F. Borgi, and A. Al- Ma’shani. 2014. Games on the seashore of Salalah: the discovery of mancala games in Dhofar, Sultanate of Oman. Arabian Archaeology and Epigraphy 25: 115– 120. Chowns, T.M., A.H. Ivester, R.L. Kath, B.K. Meyer, D.H. Thomas, and P.R. Hanson. 2014. A New Hypothesis for the Formation of the Georgia Sea Islands through the Breaching of the Silver Bluff Barrier and Dissection of the Ancestral Altamaha-Ogeechee Drainage. Abstract, 63rd Annual Meeting, Geological Society of America, Southeastern Section, April 10–11, 2014. 2 DeSalle, R., and I. Tattersall. 2014. Mr. Murray, you lose the bet.