July 16, 1968 G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DETERMINE Ol. Eg., X U.S
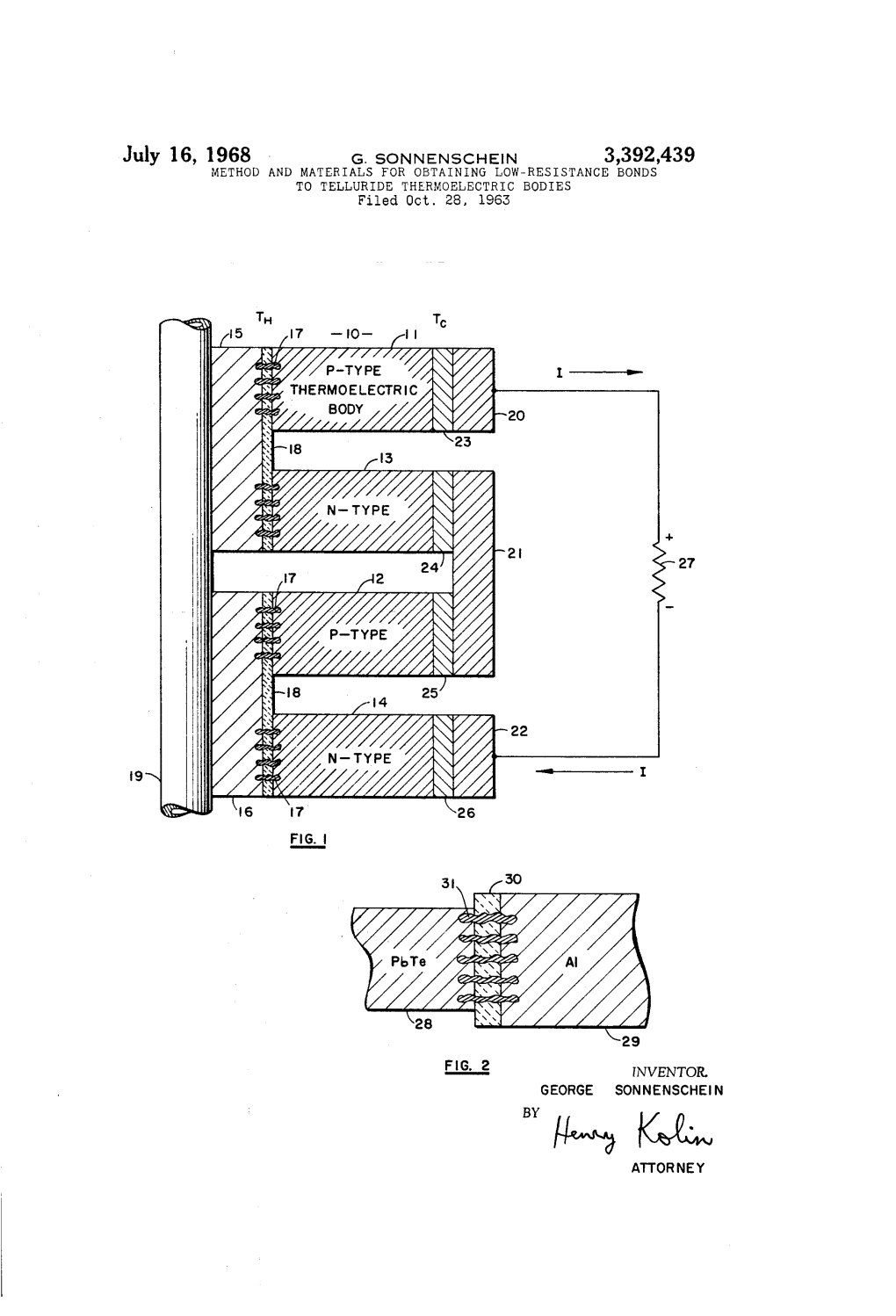
United States Patent (19. 11 4,128,338 Wong 45 Dec. 5, 1978 54 MODIFIED OPTICAL TRANSMISSION 3,725,135 4/1973 Hager et al. .......................... 148/15 TECHNIQUE FORCHARACTERIZING 3,902,924 9/1975 Maciolek et al. ..................... 148/15 EPTAXAL LAYERS Primary Examiner-John K. Corbin (75) Inventor: Theodore T. S. Wong, Maynard, Assistant Examiner-R. A. Rosenberger Mass. Attorney, Agent, or Firm-Theodore F. Neils; David R. 73) Assignee: Honeywell Inc., Minneapolis, Minn. Fairbairn (21) Appl. No: 807,608 57 ABSTRACT al An improved method of determining the energy band (22 Filed: Jun. 17, 1977 gap of an epitaxial semiconductor layer on a substrate 51 int. Cl’............................................. GON 21/22 corrects for an overestimation of energy gap yielded by 52 U.S. C. ................................................. 356/432 normal optical transmittance measurements. The over 58 Field of Search ........................ 356/201, 202,203 estimation of energy bandgap is caused by a graded (56) References Cited bandgap region which exists between the epitaxial semi U.S. PATENT DOCUMENTSw conductorductor 1layer and the substrate s 3496,024 2/1970 Ruehrwein.................. 148/33.5 3 Claims, 10 Drawing Figures DETERMINE MEASURE OPTICAL ds ded TRANSMISSION (T ) DETERMINE ol. Eg., x U.S. Patent Dec. 5, 1978 Sheet 1 of 5 4,128,338 X 2 O H () O al O U THCKNESS U.S. Patent Dec. 5, 1978 Sheet 2 of 5 4,128,338 MEASURE DETERMINE OPTICAL ds de d TRANSMISSION (T ) FIG. 4 DETERMINE c., E.g. x 2 FIG.5 20 40 60 80 IOO I2O 4O 6O 80 20O MICRONS SUBSTRATE GRADED GAP LAYER REGON U.S. -

D. I. Bletskan "Phase Equilibrium in the Binary Systems a IV
Journal of Ovonic Research Vol. 1, No. 5, October 2005, p. 53 - 60 PHASE EQUILIBRIUM IN THE SYSTEMS AIV – BVI Part. II Systems Germanium-Chalcogen D. I. Bletskan* Uzhgorod University, Uzhgorod, Ukraine The phases in the system germanium – chalcogen are reviewed. The phase transitions are discussed. 1.4 System Ge-S The phase diagram T-x of the binary system Ge-S has been firstly built by A. V. Novoselova et al. [39] using the data of thermal and X-ray analysis. Thereafter, the regions of the phase diagrams of the system Ge-S in the neighborhood of GeS and GeS2 have been carefully determined in [40-44] and are represented in Fig. 1.4. In this system there were found two stable chemical compounds, whose stoichiometric compositions correspond to GeS and GeSe2. The temperature conditions of GeS and GeS2 synthesis, the melting character and melting temperature, given in the literature, are different. Thus, the melting temperature of GeS, after the data of various authors are 938 K [39, 42], 940 K [40], 931 K [41, 46]. Many research reports [39, 42-44] show that GeS compound melts congruently. Nevertheless, in the papers [40, 41, 46] is demonstrated that, during very slow heating, GeS melts incongruently, according to the peritectic reaction: GeS(solid) = Ge(solid) + Liq(53 at.% S) (1.4) In the system Ge-S, on the germanium side, there is a large domain (3÷45 at.% S) of layer separation in the liquid state (stratification) with one phase rich in germanium and a phase with the composition approaching GeS. -

19800021154.Pdf
NEW PbSnTe HETEROJLJNCTION LASER DIODE STRUCTURES WITH IMPROVEDPERFORMANCE* § C. G. Fonstad, D. Kasemset, H. H. Hsieh , and S. Rotter Departmentof Electrical Engineeringand Computer Science -andCenter for Materials Scienceand Engineering? MassachusettsInstitute of Technology Cambridge,Massachusetts 02139 INTRODUCTION In this article, we will summarize several ofour recent advances in the state-of-the-artof lead tin telluride double heterojunction laser diodes,ad- vanceswhich make significant strides in increasing the operating temperatures ofthese devices and in controlling the modal quality and tunability of their output. CW operationto 120°K andpulsed operation to 166°K withsingle, low- est ordertransverse mode emission to in excess of four times threshold at 80°K havebeen achieved in buried stripe lasers fabricated by liquidphase epitaxy inthe lattice-matched system, lead-tin telluride-lead telluride selenide [1,2]. At the same time, liquidphase epitaxy has been used to produce PbSnTe distri- butedfeedback lasers with much broadercontinuous single mode tuningranges than are availablefrom Fabry-Perot lasers [3]. The physicsand philosophy behind these advances is as important as the structuresand performance of the specific devices embodying the advances, par- ticularlysince structures are continually being evolved and the performance continuesto be improved. There is art in anyscience, but as we will demon- strate, there is a tremendous amount ofscience to be applied to Pb-salt tun- ablediode lasers, andwhere this is done,their performance can be predicted, tailored,and reproducibly controlled. Most importantly,their performance can bedramatically enhance<. HIGH TEMPERATUREOPERATION Achievinghigher temperature operation of laser diodesrequires both that thethreshold current density be reduced at highertemperatures, and that the totalthreshold current be reduced. -

High-Throughput Physical Vapour Deposition Flexible Thermoelectric Generators
www.nature.com/scientificreports OPEN High-throughput physical vapour deposition fexible thermoelectric generators Received: 14 January 2019 Katrina A. Morgan 1, Tian Tang2, Ioannis Zeimpekis 1, Andrea Ravagli1, Chris Craig1, Accepted: 25 February 2019 Jin Yao1, Zhuo Feng1, Dmitry Yarmolich3, Clara Barker 2, Hazel Assender2 & Published: xx xx xxxx Daniel W. Hewak1 Flexible thermoelectric generators (TEGs) can provide uninterrupted, green energy from body-heat, overcoming bulky battery confgurations that limit the wearable-technologies market today. High- throughput production of fexible TEGs is currently dominated by printing techniques, limiting material choices and performance. This work investigates the compatibility of physical vapour deposition (PVD) techniques with a fexible commercial process, roll-to-roll (R2R), for thermoelectric applications. We demonstrate, on a fexible polyimide substrate, a sputtered Bi2Te3/GeTe TEG with Seebeck coefcient (S) of 140 μV/K per pair and output power (P) of 0.4 nW per pair for a 20 °C temperature diference. For the frst time, thermoelectric properties of R2R sputtered Bi2Te3 flms are reported and we demonstrate the ability to tune the power factor by lowering run times, lending itself to a high- speed low-cost process. To further illustrate this high-rate PVD/R2R compatibility, we fabricate a TEG using Virtual Cathode Deposition (VCD), a novel high deposition rate PVD tool, for the frst time. This Bi2Te3/Bi0.5Sb1.5Te3 TEG exhibits S = 250 μV/K per pair and P = 0.2 nW per pair for a 20 °C temperature diference. Termoelectric generators (TEGs) can provide constant power for fexible electronic platforms. Using the body’s warmth, they do not rely on solar power, unlike photovoltaic generators, or on the user’s ftness, unlike elec- tromagnetic induction generators. -

WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/DK20 15/050343 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 11 November 2015 ( 11. 1 1.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: PA 2014 00655 11 November 2014 ( 11. 1 1.2014) DK (84) Designated States (unless otherwise indicated, for every 62/077,933 11 November 2014 ( 11. 11.2014) US kind of regional protection available): ARIPO (BW, GH, 62/202,3 18 7 August 2015 (07.08.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: LUNDORF PEDERSEN MATERIALS APS TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [DK/DK]; Nordvej 16 B, Himmelev, DK-4000 Roskilde DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (DK). -

Design and Analysis of Novel Ge-Gete PN Junction for Photovoltaics Andrew M
Marquette University e-Publications@Marquette Electrical and Computer Engineering Faculty Electrical and Computer Engineering, Department Research and Publications of 7-25-2016 Design and Analysis of Novel Ge-GeTe PN Junction for Photovoltaics Andrew M. Jones Air Force Institute of Technology Ronald A. Coutu Jr. Marquette University, [email protected] Robert A. Lake Air Force Institute of Technology Published version. 2016 IEEE National Aerospace and Electronics Conference (NAECON) and Ohio Innovation Summit (OIS), (July 25-29, 2016). DOI. U.S. Government work not protected by U.S. copyright. Ronald A. Coutu, Jr. was affiliated with the Air Force Institute of Technology at the time of publication. Design and Analysis of Novel Ge-GeTe PN Junction for Photovoltaics Andrew M. Jones1, Ronald A. Coutu Jr., Robert A. Lake Department of Electrical and Computer Engineering Air Force Institute of Technology Wright Patterson AFB, OH, USA andrew.jones@afit.edu Abstract—The continuing rise in demand for energy places devices, little attention has been placed on the previous a similarly increasing demand to improve power production precursor to the semiconductor age, Ge. methods and efficiency. In regards to solar power generation, one One particular ChG consisting of Ge is germanium telluride major limiting factor with existing photovoltaic (PV) systems is the management of heat produced and photon interactions with (GeTe). GeTe has long since been studied for its unique the PV device. Typical devices operate within the 300-1000 nm amorphous-crystalline transitioning behavior with change in range of the solar spectrum, greatly limiting the range of photons temperature. In earlier works it was observed that ratios of used for power generation. -

Partial Pressures and High-Temperature Thermodynamic Properties for the Germanium-Tellurium System
Marquette University e-Publications@Marquette Mechanical Engineering Faculty Research and Publications Mechanical Engineering, Department of 4-2019 Partial Pressures and High-Temperature Thermodynamic Properties for the Germanium-Tellurium System Robert F. Brebrick Marquette University, [email protected] Follow this and additional works at: https://epublications.marquette.edu/mechengin_fac Part of the Mechanical Engineering Commons Recommended Citation Brebrick, Robert F., "Partial Pressures and High-Temperature Thermodynamic Properties for the Germanium-Tellurium System" (2019). Mechanical Engineering Faculty Research and Publications. 265. https://epublications.marquette.edu/mechengin_fac/265 Marquette University e-Publications@Marquette Mechanical Engineering Faculty Research and Publications/College of Engineering This paper is NOT THE PUBLISHED VERSION. Access the published version via the link in the citation below. Journal of Phase Equilibria & Diffusion, Vol. 40, No. 2 (April 2019): 291-305. DOI. This article is © Springer and permission has been granted for this version to appear in e-Publications@Marquette. Springer does not grant permission for this article to be further copied/distributed or hosted elsewhere without express permission from Springer. Partial Pressures and High-Temperature Thermodynamic Properties for the Germanium-Tellurium System Robert F. Brebrick Department of Mechanical Engineering, Marquette University, Milwaukee, WI, 53233, USA Abstract Our earlier optical density measurements over Ge Te compositions are reanalyzed. Spectral characteristics of the GeTe(g) spectrum are determined from measurements with a 1 at.% Te sample near 1200 K. Beers law constants for GeTe(g) are established by requiring− consistency between spectroscopic results for the dissociation of GeTe(g) into Ge(g) and Te (g) and our description in terms of the partial pressure of Te and the partial optical density of GeTe over Ge saturated GeTe(c). -
![Dominant Point Defects in Germanium Telluride Crystals [ ] [ ] [ ] [ ] Te](https://docslib.b-cdn.net/cover/0310/dominant-point-defects-in-germanium-telluride-crystals-te-2260310.webp)
Dominant Point Defects in Germanium Telluride Crystals [ ] [ ] [ ] [ ] Te
Chem. Met. Alloys 5 (2012) 155-159 Ivan Franko National University of Lviv www.chemetal-journal.org Dominant point defects in germanium telluride crystals Dmytro FREIK 1, Igor GORICHOK 1*, Liubov YURCHYSHYN 1 1 Institute of Physics and Chemistry, Precarpathian National Vasyl Stefanyk University, Shevchenka St. 57, 76018 Ivano-Frankivsk, Ukraine * Corresponding author. Tel.: +380-34-2596082; e-mail: [email protected] Received December 10, 2012; accepted December 26, 2012; available on-line July 5, 2013 The features of the experimental dependences of the concentration of charge carriers on the temperature and chemical composition of germanium telluride with NaCl-type structure at temperatures T = 550-850 K and concentrations of excess tellurium XTe = 0.01-0.1 at.% Te are interpreted, and a crystal chemical model is proposed for the defect subsystem. It was found that the dominant defects under these conditions are doubly ionized metal vacancies, which define the character of the dependences p(T), p(XTe ). At temperatures above 750 K and for excess tellurium concentrations above 0.04 at.% Te, also antistructural chalcogen atoms have a significant impact on the concentration of free holes. The concentrations of other defects are much lower and do not affect the electrical properties of the material. Germanium telluride / Electrical properties / Point defects Introduction antistructural defects on the electrical properties is controversial [3-7] . In most studies, the conclusions Semiconductor compounds IV-VI and solid on the predominant type of defects are made based on solutions based on them are basic materials for indirect experimental measurements, which are not modern infrared electronics and are used to create always unambiguously interpreted. -

Band Crossing Evidence in Pbsnte Observed by Optical Transmission
Brazilian Journal of Physics, vol. 29, no. 4, Decemb er, 1999 771 Band Crossing Evidence in PbSnTe Observed by Optical Transmission Measurements 1 2 2 2 2 S. O. Ferreira , E. Abramof ,P. Motisuke ,P. H. O. Rappl , H. Closs , 2 2 2 A. Y. Ueta , C. Boschetti , and I. N. Bandeira , 1: Dep. F sica, UniversidadeFederal de Vicosa, 36571-000, Vicosa, MG, Brazil 2: Instituto Nacional de Pesquisas Espaciais, C.P. 515 - 12201-970 S~ao Jos e dos Campos, SP, Brazil Received February 8, 1999 Using high quality epitaxial layers, wehave obtained direct evidence of the band inversion in the Pb Sn Te system. The samples, covering the whole comp osition range, were grown by 1x x molecular b eam epitaxy on 111BaF substrates. A minimum in the resistivity as a function 2 of temp erature was observed for all samples with Sn comp osition 0:35 x 0:70. In the same samples and at the same temp erature, temp erature dep endent optical transmission measurements have revealed a change in signal of the energy gap temp erature derivative, a direct evidence of the band inversion. However, the temp erature for which the inversion o ccurs is not the one exp ected by the band inversion mo del. This discrepancy is supp osed to b e due to the Burstein-Moss shift caused by the relatively high hole concentration observed in these samples. band edge states inverted, up to the SnT e value. The I Intro duction Sn comp osition for which the band inversion should Lead-tin telluride is a narrow gap semiconductor which o ccur varies from x 0:35 to x 0:70 as the tem- have b een investigated for manyyears and applied p erature increases from 4 to 300 K. -

United States Patent (19) 11) Patent Number: 4,722,087 Partin 45) Date of Patent: Jan
United States Patent (19) 11) Patent Number: 4,722,087 Partin 45) Date of Patent: Jan. 26, 1988 (54) LEAD-STRONTUM-CHALCOGENIDE Grown by Molecular Beam Epitaxy"; Journal of Elec DODE LASER tronic, vol. 13, No. 3, pp. 493-504, May 1984. (75 Inventor: Dale L. Partin, Sterling Heights, Primary Examiner-William L. Sikes Mich. Assistant Examiner-Bertha Randolph 73) Assignee: General Motors Corporation, Detroit, Attorney, Agent, or Firm-Randy M. Tung Mich. 57 ABSTRACT (21) Appl. No.: 895,286 A double heterojunction lead salt infrared diode laser 22) Filed: Aug. 11, 1986 having an active region layer of a lead salt semiconduc (51) Int. Cl."................................................ H01S 3/19 tor of a given lattice constant, energy band gap, and 52) U.S. C. ........................................ 372/44; 372/45; index of refraction. The active region layer is sand 357/16; 357/17; 357/61 wiched between two lead salt semiconductor layers (58) Field of Search ...................... 372/44, 45; 357/16, containing strontium and selenium that are mutually of 357/63, 17, 61 opposite conductivity type and have substantially the same lattice constant as the active region layer. In addi (56) References Cited tion, the two outside layers have an energy band gap U.S. PATENT DOCUMENTS greater than the active region layer and an index of 4,608,694 8/1986 Partin .................................... 372/44 refraction less than the active region layer. The result 4,612,644 9/1986 Partin .................................... 372/44 ing laser has lattice matching, as well as enhanced car OTHER PUBLICATIONS rier confinement and optical confinement. D. L. Partin; "Lead-Europium-Selenide-Telluride 3 Claims, 1 Drawing Figure on Junction sS.s U.S. -

Monday Afternoon, October 22, 2018
Monday Afternoon, October 22, 2018 Si. The RMS roughness of the ALD MoSix film on the dry-cleaned Si was 2.26nm while on the HF cleaned Si the RMS roughness was 2.78nm. This Electronic Materials and Photonics Division shows that the dry clean developed in this study is capable of producing Room 101A - Session EM+AM+NS+PS-MoA cleaner and smoother Si surfaces than the traditional aqueous HF clean. Atomic Layer Processing: Selective-Area Patterning 2:00pm EM+AM+NS+PS-MoA-3 Probing Strategies for Selective Deposition that Exploit Competitive Interactions, James Engstrom, Cornell (Assembly/Deposition/Etching) University INVITED Moderators: Michael Filler, Georgia Institute of Technology, Jessica Hilton, Selective thin film processes, including atomic layer deposition, have the RHK Technology potential to enable next-generation manufacturing and patterning at the 5 1:20pm EM+AM+NS+PS-MoA-1 Area-Selective Deposition of Crystalline nm node and beyond, with direct applications in the nanofabrication of Perovskites, E Lin, Brennan Coffey, Z Zhang, P Chen, B Edmondson, J functional layers such as gate dielectrics, metal contacts, and Ekerdt, University of Texas at Austin capping/barrier layers. Well-known for its ability to deposit atomically thin Epitaxial growth of crystalline perovskites enables opportunities in films with Å-scale precision along the growth direction and conformally integrating perovskite properties into electronic and photonic devices. over complex 3D substrates, atomic layer deposition (ALD) has emerged as Pattern definition is a necessary step in many device applications and a key nanomanufacturing process. In this regard, the range and scope of definition through etching can be problematic with titanium-based ALD-based applications and capabilities can be substantially extended by perovskites. -

United States Patent [191 4,612,644
United States Patent [191 [11] Patent Number: 4,612,644 Partin [45] Date of Patent: Sep. 16, 1986 [54] LEAD-ALLOY-TELLURIDE by Molecular Beam Epitaxy”, Journal of Electronics HETEROJUNCI‘ION SEMICONDUCTOR Materials, vol. 13, No. 3, 1984. LASER Weber et al., “Waveguide and Luminescent Properties [75] Inventor: Dale L. Partin, Sterling Heights, of Thin Film Pb-Salt Injection Lasers”, Journal Applied Mich. Physics, vol. 44, No. 11, Nov. 1973, pp. 4991-5000. [73] Assignee: General Motors Corporation, Detroit, Primary Examiner-James W. Davie Mich. Assistant Examiner—-Georgia Y. Epps Attorney, Agent, or Firm—Randy W. Tung [21] Appl. No.: 754,171 [57] ABSTRACT [22] Filed: Jul. 12, 1985 A double heterojunction lead salt infrared diode laser [51] Int. Clx‘ ....................... .. H018 3/19; H01L 33/00 having an active region layer of a lead salt semiconduc [52] US. Cl. ...................................... .. 372/44; 357/16; tor of a given lattice constant, energy band gap, and 357/17; 357/61; 372/45 index of refraction. The active region layer is sand [58] Field of Search .................... .. 372/44, 45; 357/ 17, wiched between two lead salt semiconductor layers 357/61, 16 containing calcium and one element selected from the [56] References Cited group consisting of europium and strontium that are mutually of opposite conductivity type and have sub PUBLICATIONS stantially the same lattice constant as the active region Partin et al., “Wavelength coverage of Lead-Europi layer. In addition, the two outside layers have an energy um-Selenide-Telluride Diode Lasers”, Applied Physics band gap greater than the active region layer and an Letters, 45(3), Aug.