High-Resolution Genome-Wide Mapping of Genetic Alterations in Human Glial Brain Tumors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Structure and Function of the Human Recq DNA Helicases
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2005 Structure and function of the human RecQ DNA helicases Garcia, P L Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-34420 Dissertation Published Version Originally published at: Garcia, P L. Structure and function of the human RecQ DNA helicases. 2005, University of Zurich, Faculty of Science. Structure and Function of the Human RecQ DNA Helicases Dissertation zur Erlangung der naturwissenschaftlichen Doktorw¨urde (Dr. sc. nat.) vorgelegt der Mathematisch-naturwissenschaftlichen Fakultat¨ der Universitat¨ Z ¨urich von Patrick L. Garcia aus Unterseen BE Promotionskomitee Prof. Dr. Josef Jiricny (Vorsitz) Prof. Dr. Ulrich H ¨ubscher Dr. Pavel Janscak (Leitung der Dissertation) Z ¨urich, 2005 For my parents ii Summary The RecQ DNA helicases are highly conserved from bacteria to man and are required for the maintenance of genomic stability. All unicellular organisms contain a single RecQ helicase, whereas the number of RecQ homologues in higher organisms can vary. Mu- tations in the genes encoding three of the five human members of the RecQ family give rise to autosomal recessive disorders called Bloom syndrome, Werner syndrome and Rothmund-Thomson syndrome. These diseases manifest commonly with genomic in- stability and a high predisposition to cancer. However, the genetic alterations vary as well as the types of tumours in these syndromes. Furthermore, distinct clinical features are observed, like short stature and immunodeficiency in Bloom syndrome patients or premature ageing in Werner Syndrome patients. Also, the biochemical features of the human RecQ-like DNA helicases are diverse, pointing to different roles in the mainte- nance of genomic stability. -

Open Full Page
CCR PEDIATRIC ONCOLOGY SERIES CCR Pediatric Oncology Series Recommendations for Childhood Cancer Screening and Surveillance in DNA Repair Disorders Michael F. Walsh1, Vivian Y. Chang2, Wendy K. Kohlmann3, Hamish S. Scott4, Christopher Cunniff5, Franck Bourdeaut6, Jan J. Molenaar7, Christopher C. Porter8, John T. Sandlund9, Sharon E. Plon10, Lisa L. Wang10, and Sharon A. Savage11 Abstract DNA repair syndromes are heterogeneous disorders caused by around the world to discuss and develop cancer surveillance pathogenic variants in genes encoding proteins key in DNA guidelines for children with cancer-prone disorders. Herein, replication and/or the cellular response to DNA damage. The we focus on the more common of the rare DNA repair dis- majority of these syndromes are inherited in an autosomal- orders: ataxia telangiectasia, Bloom syndrome, Fanconi ane- recessive manner, but autosomal-dominant and X-linked reces- mia, dyskeratosis congenita, Nijmegen breakage syndrome, sive disorders also exist. The clinical features of patients with DNA Rothmund–Thomson syndrome, and Xeroderma pigmento- repair syndromes are highly varied and dependent on the under- sum. Dedicated syndrome registries and a combination of lying genetic cause. Notably, all patients have elevated risks of basic science and clinical research have led to important in- syndrome-associated cancers, and many of these cancers present sights into the underlying biology of these disorders. Given the in childhood. Although it is clear that the risk of cancer is rarity of these disorders, it is recommended that centralized increased, there are limited data defining the true incidence of centers of excellence be involved directly or through consulta- cancer and almost no evidence-based approaches to cancer tion in caring for patients with heritable DNA repair syn- surveillance in patients with DNA repair disorders. -

Age Dependence of Tumor Genetics in Unfavorable
Cetinkaya et al. BMC Cancer 2013, 13:231 http://www.biomedcentral.com/1471-2407/13/231 RESEARCH ARTICLE Open Access Age dependence of tumor genetics in unfavorable neuroblastoma: arrayCGH profiles of 34 consecutive cases, using a Swedish 25-year neuroblastoma cohort for validation Cihan Cetinkaya1,2, Tommy Martinsson3, Johanna Sandgren1,4, Catarina Träger5, Per Kogner5, Jan Dumanski1, Teresita Díaz de Ståhl1,4† and Fredrik Hedborg1,6*† Abstract Background: Aggressive neuroblastoma remains a significant cause of childhood cancer death despite current intensive multimodal treatment protocols. The purpose of the present work was to characterize the genetic and clinical diversity of such tumors by high resolution arrayCGH profiling. Methods: Based on a 32K BAC whole-genome tiling path array and using 50-250K Affymetrix SNP array platforms for verification, DNA copy number profiles were generated for 34 consecutive high-risk or lethal outcome neuroblastomas. In addition, age and MYCN amplification (MNA) status were retrieved for 112 unfavorable neuroblastomas of the Swedish Childhood Cancer Registry, representing a 25-year neuroblastoma cohort of Sweden, here used for validation of the findings. Statistical tests used were: Fisher’s exact test, Bayes moderated t-test, independent samples t-test, and correlation analysis. Results: MNA or segmental 11q loss (11q-) was found in 28/34 tumors. With two exceptions, these aberrations were mutually exclusive. Children with MNA tumors were diagnosed at significantly younger ages than those with 11q- tumors (mean: 27.4 vs. 69.5 months; p=0.008; n=14/12), and MNA tumors had significantly fewer segmental chromosomal aberrations (mean: 5.5 vs. 12.0; p<0.001). -

Predicting Gene Ontology Biological Process from Temporal Gene Expression Patterns Astrid Lægreid,1,4 Torgeir R
Methods Predicting Gene Ontology Biological Process From Temporal Gene Expression Patterns Astrid Lægreid,1,4 Torgeir R. Hvidsten,2 Herman Midelfart,2 Jan Komorowski,2,3,4 and Arne K. Sandvik1 1Department of Cancer Research and Molecular Medicine, Norwegian University of Science and Technology, N-7489 Trondheim, Norway; 2Department of Information and Computer Science, Norwegian University of Science and Technology, N-7491 Trondheim, Norway; 3The Linnaeus Centre for Bioinformatics, Uppsala University, SE-751 24 Uppsala, Sweden The aim of the present study was to generate hypotheses on the involvement of uncharacterized genes in biological processes. To this end,supervised learning was used to analyz e microarray-derived time-series gene expression data. Our method was objectively evaluated on known genes using cross-validation and provided high-precision Gene Ontology biological process classifications for 211 of the 213 uncharacterized genes in the data set used. In addition,new roles in biological process were hypothesi zed for known genes. Our method uses biological knowledge expressed by Gene Ontology and generates a rule model associating this knowledge with minimal characteristic features of temporal gene expression profiles. This model allows learning and classification of multiple biological process roles for each gene and can predict participation of genes in a biological process even though the genes of this class exhibit a wide variety of gene expression profiles including inverse coregulation. A considerable number of the hypothesized new roles for known genes were confirmed by literature search. In addition,many biological process roles hypothesi zed for uncharacterized genes were found to agree with assumptions based on homology information. -

Fanconi Anemia, Bloom Syndrome and Breast Cancer
A multiprotein complex in DNA damage response network of Fanconi anemia, Bloom syndrome and Breast cancer Weidong Wang Lab of Genetics, NIA A Multi-protein Complex Connects Two Genomic Instability Diseases: Bloom Syndrome and Fanconi Anemia Bloom Syndrome . Genomic Instability: -sister-chromatid exchange . Cancer predisposition . Mutation in BLM, a RecQ DNA Helicase . BLM participates in: HR-dependent DSB repair Recovery of stalled replication forks . BLM works with Topo IIIa and RMI to Suppress crossover recombination Courtesy of Dr. Ian Hickson A Multi-protein Complex Connects Two Genomic Instability Diseases: Bloom Syndrome and Fanconi Anemia P I l o r t n o BLM IP kDa C HeLa BLAP 250 Nuclear Extract 200- BLM* FANCA* 116- TOPO IIIα* 97- BLAP 100 MLH1* BLM IP BLAP 75 * 66- RPA 70 IgG H 45- * 30- RPA32 IgG L 20- * 12- RPA14 Meetei et al. MCB 2003 A Multi-protein Complex Connects Two Genomic Instability Diseases: Bloom Syndrome and Fanconi Anemia P I A C N A F BLM IP HeLa FANCM= FAAP 250 BLAP 250 Nuclear Extract BLM* BLM* * FANCA* FANCA TOPO IIIα* TOPO IIIα* FAAP 100 BLAP 100 FANCB= FAAP 95 MLH1 FANCA IP BLM IP BLAP 75 BLAP 75 RPA70*/FANCG* RPA 70* FANCC*/FANCE* IgG H FANCL= FAAP 43 FANCF* RPA32* IgG L Meetei et al. MCB 2003 Meetei et al. Nat Genet. 2003, 2004, 2005 BRAFT-a Multisubunit Machine that Maintains Genome Stability and is defective in Fanconi anemia and Bloom syndrome BRAFT Super-complex Fanconi Anemia Bloom Syndrome Core Complex Complex 12 polypeptides 7 polypeptides FANCA BLM Helicase (HJ, fork, D-loop), fork FANCC regression, dHJ dissolution Topo IIIα Topoisomerase, FANCE dHJ dissolution FANCF BLAP75 RMI1 FANCG Stimulates dHJ dissolution. -

Targeting PH Domain Proteins for Cancer Therapy
The Texas Medical Center Library DigitalCommons@TMC The University of Texas MD Anderson Cancer Center UTHealth Graduate School of The University of Texas MD Anderson Cancer Biomedical Sciences Dissertations and Theses Center UTHealth Graduate School of (Open Access) Biomedical Sciences 12-2018 Targeting PH domain proteins for cancer therapy Zhi Tan Follow this and additional works at: https://digitalcommons.library.tmc.edu/utgsbs_dissertations Part of the Bioinformatics Commons, Medicinal Chemistry and Pharmaceutics Commons, Neoplasms Commons, and the Pharmacology Commons Recommended Citation Tan, Zhi, "Targeting PH domain proteins for cancer therapy" (2018). The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access). 910. https://digitalcommons.library.tmc.edu/utgsbs_dissertations/910 This Dissertation (PhD) is brought to you for free and open access by the The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences at DigitalCommons@TMC. It has been accepted for inclusion in The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access) by an authorized administrator of DigitalCommons@TMC. For more information, please contact [email protected]. TARGETING PH DOMAIN PROTEINS FOR CANCER THERAPY by Zhi Tan Approval page APPROVED: _____________________________________________ Advisory Professor, Shuxing Zhang, Ph.D. _____________________________________________ -

DNA Repair As an Emerging Target for COPD-Lung Cancer Overlap Catherine R
DNA Repair as an Emerging Target for COPD-Lung Cancer Overlap Catherine R. Sears1 1Division of Pulmonary, Critical Care, Sleep and Occupational Medicine, Department of Medicine, Indiana University, Indianapolis, Indiana; The Richard L. Roudebush Veterans Affairs Medical Center, Indianapolis, IN, 46202, U.S.A. Corresponding Author: Catherine R. Sears, M.D. 980 W. Walnut Street Walther Hall, C400 Indianapolis, IN 46202 tel: 317-278-0413. fax: 317-278-7030 [email protected] Abstract length: 151 Article length (excluding tables and figures): 3,988 Number of Figures: 1 Tables: 1 Conflict of Interest Declaration: The author of this publication has no conflicts of interest to declare. This publication is supported in part by funding from the American Cancer Society (128511-MRSG-15-163-01- DMC) and the Showalter Research Foundation. ____________________________________________________ This is the author's manuscript of the article published in final edited form as: Sears, C. R. (2019). DNA repair as an emerging target for COPD-lung cancer overlap. Respiratory Investigation, 57(2), 111–121. https://doi.org/10.1016/j.resinv.2018.11.005 Abstract Cigarette smoking is the leading cause of lung cancer and chronic obstructive pulmonary disease (COPD). Many of the detrimental effects of cigarette smoke have been attributed to the development of DNA damage, either directly from chemicals contained in cigarette smoke or as a product of cigarette smoke-induced inflammation and oxidative stress. In this review, we discuss the environmental, epidemiological, and physiological links between COPD and lung cancer and the likely role of DNA damage and repair in COPD and lung cancer development. -

Distinct Roles of BRCA2 in Replication Fork Protection in Response to Hydroxyurea and DNA Interstrand Cross-Links
Downloaded from genesdev.cshlp.org on October 1, 2021 - Published by Cold Spring Harbor Laboratory Press Distinct roles of BRCA2 in replication fork protection in response to hydroxyurea and DNA interstrand cross-links Kimberly A. Rickman,1 Raymond J. Noonan,1 Francis P. Lach,1 Sunandini Sridhar,1 Anderson T. Wang,1,5 Avinash Abhyankar,2 Athena Huang,1 Michael Kelly,3 Arleen D. Auerbach,4 and Agata Smogorzewska1 1Laboratory of Genome Maintenance, The Rockefeller University, New York, New York 10065, USA; 2New York Genome Center, New York, New York 10013, USA; 3Tufts Medical Center, Boston, Massachusetts 02111, USA; 4Human Genetics and Hematology, The Rockefeller University, New York, New York 10065, USA DNA interstrand cross-links (ICLs) are a form of DNA damage that requires the interplay of a number of repair proteins including those of the Fanconi anemia (FA) and the homologous recombination (HR) pathways. Pathogenic variants in the essential gene BRCA2/FANCD1, when monoallelic, predispose to breast and ovarian cancer, and when biallelic, result in a severe subtype of Fanconi anemia. BRCA2 function in the FA pathway is attributed to its role as a mediator of the RAD51 recombinase in HR repair of programmed DNA double-strand breaks (DSB). BRCA2 and RAD51 functions are also required to protect stalled replication forks from nucleolytic degradation during re- sponse to hydroxyurea (HU). While RAD51 has been shown to be necessary in the early steps of ICL repair to prevent aberrant nuclease resection, the role of BRCA2 in this process has not been described. Here, based on the analysis of BRCA2 DNA-binding domain (DBD) mutants (c.8488-1G>A and c.8524C>T) discovered in FA patients presenting with atypical FA-like phenotypes, we establish that BRCA2 is necessary for the protection of DNA at ICLs. -

UNIVERSITY of CALIFORNIA Los Angeles Molecular and Genetic
UNIVERSITY OF CALIFORNIA Los Angeles Molecular and Genetic Study of Human Liposarcoma A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Molecular and Medical Pharmacology by Kathleen Barzan Smith 2012 ABSTRACT OF THE DISSERTATION Molecular and Genetic Study of Human Liposarcoma by Kathleen Barzan Smith Doctor of Philosophy in Molecular and Medical Pharmacology University of California, Los Angeles, 2012 Professor Frederick C. Eilber, Co-chair Professor Hong Wu, Co-chair Sarcomas are cancers of connective tissue, such as bone, nerves, and muscle. Liposarcoma, a neoplasm arising within adipose tissue, is the most common soft tissue sarcoma. Although most commonly found in the retroperitoneum or thighs, liposarcomas can arise throughout the body and are often large when found. Current treatment is limited to surgery and radiation, with chemotherapy doing little to improve prognosis in advanced cases. D ue to the large size of tumors and t heir proximity to organs and healthy tissue, complete surgical removal is difficult and recurrence rates remain high. Liposarcoma can be divided into three histological subtypes: pleomorphic, myxoid/round cell, and /well- dedifferentiated. Here we demonstrate the generation of three novel dedifferentiated liposarcoma xenograft models from freshly resected patient tissue. These xenograft models and their derived cultured cells successfully recapitulate the morphological and gene expression profiles of their patient tumors throughout serial passage in mice. Interestingly, the patients whose tumors could engraft and be serially passaged had significantly shorter survival than patients whose tumors did not engraft. These ii tumors carried gene expression signatures with more aggressive and less differentiated features. -

Nº Ref Uniprot Proteína Péptidos Identificados Por MS/MS 1 P01024
Document downloaded from http://www.elsevier.es, day 26/09/2021. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Nº Ref Uniprot Proteína Péptidos identificados 1 P01024 CO3_HUMAN Complement C3 OS=Homo sapiens GN=C3 PE=1 SV=2 por 162MS/MS 2 P02751 FINC_HUMAN Fibronectin OS=Homo sapiens GN=FN1 PE=1 SV=4 131 3 P01023 A2MG_HUMAN Alpha-2-macroglobulin OS=Homo sapiens GN=A2M PE=1 SV=3 128 4 P0C0L4 CO4A_HUMAN Complement C4-A OS=Homo sapiens GN=C4A PE=1 SV=1 95 5 P04275 VWF_HUMAN von Willebrand factor OS=Homo sapiens GN=VWF PE=1 SV=4 81 6 P02675 FIBB_HUMAN Fibrinogen beta chain OS=Homo sapiens GN=FGB PE=1 SV=2 78 7 P01031 CO5_HUMAN Complement C5 OS=Homo sapiens GN=C5 PE=1 SV=4 66 8 P02768 ALBU_HUMAN Serum albumin OS=Homo sapiens GN=ALB PE=1 SV=2 66 9 P00450 CERU_HUMAN Ceruloplasmin OS=Homo sapiens GN=CP PE=1 SV=1 64 10 P02671 FIBA_HUMAN Fibrinogen alpha chain OS=Homo sapiens GN=FGA PE=1 SV=2 58 11 P08603 CFAH_HUMAN Complement factor H OS=Homo sapiens GN=CFH PE=1 SV=4 56 12 P02787 TRFE_HUMAN Serotransferrin OS=Homo sapiens GN=TF PE=1 SV=3 54 13 P00747 PLMN_HUMAN Plasminogen OS=Homo sapiens GN=PLG PE=1 SV=2 48 14 P02679 FIBG_HUMAN Fibrinogen gamma chain OS=Homo sapiens GN=FGG PE=1 SV=3 47 15 P01871 IGHM_HUMAN Ig mu chain C region OS=Homo sapiens GN=IGHM PE=1 SV=3 41 16 P04003 C4BPA_HUMAN C4b-binding protein alpha chain OS=Homo sapiens GN=C4BPA PE=1 SV=2 37 17 Q9Y6R7 FCGBP_HUMAN IgGFc-binding protein OS=Homo sapiens GN=FCGBP PE=1 SV=3 30 18 O43866 CD5L_HUMAN CD5 antigen-like OS=Homo -
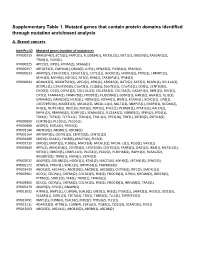
Supplementary Table 1. Mutated Genes That Contain Protein Domains Identified Through Mutation Enrichment Analysis
Supplementary Table 1. Mutated genes that contain protein domains identified through mutation enrichment analysis A. Breast cancers InterPro ID Mutated genes (number of mutations) IPR000219 ARHGEF4(2), ECT2(1), FARP1(1), FLJ20184(1), MCF2L2(1), NET1(1), OBSCN(5), RASGRF2(2), TRAD(1), VAV3(1) IPR000225 APC2(2), JUP(1), KPNA5(2), SPAG6(1) IPR000357 ARFGEF2(2), CMYA4(1), DRIM(2), JUP(1), KPNA5(2), PIK3R4(1), SPAG6(1) IPR000533 AKAP9(2), C10orf39(1), C20orf23(1), CUTL1(1), HOOK1(1), HOOK3(1), KTN1(2), LRRFIP1(3), MYH1(3), MYH9(2), NEF3(1), NF2(1), RSN(1), TAX1BP1(1), TPM4(1) IPR000694 ADAM12(3), ADAMTS19(1), APC2(2), APXL(1), ARID1B(1), BAT2(2), BAT3(1), BCAR1(1), BCL11A(2), BCORL1(1), C14orf155(3), C1orf2(1), C1QB(1), C6orf31(1), C7orf11(1), CD2(1), CENTD3(3), CHD5(3), CIC(3), CMYA1(2), COL11A1(3), COL19A1(2), COL7A1(3), DAZAP1(1), DBN1(3), DVL3(1), EIF5(1), FAM44A(1), FAM47B(1), FHOD1(1), FLJ20584(1), G3BP2(2), GAB1(2), GGA3(1), GLI1(3), GPNMB(2), GRIN2D(3), HCN3(1), HOXA3(2), HOXA4(1), IRS4(1), KCNA5(1), KCNC2(1), LIP8(1), LOC374955(1), MAGEE1(2), MICAL1(2), MICAL‐L1(1), MLLT2(1), MMP15(1), N4BP2(1), NCOA6(2), NHS(1), NUP214(3), ODZ1(3), PER1(2), PER2(1), PHC1(1), PLXNB1(1), PPM1E(2), RAI17(2), RAPH1(2), RBAF600(2), SCARF2(1), SEMA4G(1), SLC16A2(1), SORBS1(1), SPEN(2), SPG4(1), TBX1(1), TCF1(2), TCF7L1(1), TESK1(1), THG‐1(1), TP53(18), TRIF(1), ZBTB3(2), ZNF318(2) IPR000909 CENTB1(2), PLCB1(1), PLCG1(1) IPR000998 AEGP(3), EGFL6(2), PRSS7(1) IPR001140 ABCB10(2), ABCB6(1), ABCB8(2) IPR001164 ARFGAP3(1), CENTB1(2), CENTD3(3), CENTG1(2) IPR001589 -

Regulation of COX Assembly and Function by Twin CX9C Proteins—Implications for Human Disease
cells Review Regulation of COX Assembly and Function by Twin CX9C Proteins—Implications for Human Disease Stephanie Gladyck 1, Siddhesh Aras 1,2, Maik Hüttemann 1 and Lawrence I. Grossman 1,2,* 1 Center for Molecular Medicine and Genetics, Wayne State University School of Medicine, Detroit, MI 48201, USA; [email protected] (S.G.); [email protected] (S.A.); [email protected] (M.H.) 2 Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, Maryland and Detroit, MI 48201, USA * Correspondence: [email protected] Abstract: Oxidative phosphorylation is a tightly regulated process in mammals that takes place in and across the inner mitochondrial membrane and consists of the electron transport chain and ATP synthase. Complex IV, or cytochrome c oxidase (COX), is the terminal enzyme of the electron transport chain, responsible for accepting electrons from cytochrome c, pumping protons to contribute to the gradient utilized by ATP synthase to produce ATP, and reducing oxygen to water. As such, COX is tightly regulated through numerous mechanisms including protein–protein interactions. The twin CX9C family of proteins has recently been shown to be involved in COX regulation by assisting with complex assembly, biogenesis, and activity. The twin CX9C motif allows for the import of these proteins into the intermembrane space of the mitochondria using the redox import machinery of Mia40/CHCHD4. Studies have shown that knockdown of the proteins discussed in this review results in decreased or completely deficient aerobic respiration in experimental models ranging from yeast to human cells, as the proteins are conserved across species.