TILAPIA AS FOOD FISH: ENHANCEMENT of Ω-3 POLYUNSATURATED FATTY ACIDS in TILAPIA (Oreochromis Spp.)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cosmetic Creams Cosmetic Creams
Cosmetic Creams Cosmetic Creams Development, Manufacture and Marketing of Effective Skin Care Products Wilfried Rähse Author All books published by Wiley-VCH are carefully produced. Nevertheless, Dr. Wilfried Rähse authors, editors, and publisher do not Bahlenstr. 168 warrant the information contained in 40589 Düsseldorf these books, including this book, to Germany be free of errors. Readers are advised to keep in mind that statements, data, Cover Images: © keng88/Shutterstock, illustrations, procedural details or other © Arthur S. Aubry/Getty Images items may inadvertently be inaccurate. Library of Congress Card No.: applied for British Library Cataloguing-in-Publication Data A catalogue record for this book is available from the British Library. Bibliographic information published by the Deutsche Nationalbibliothek The Deutsche Nationalbibliothek lists this publication in the Deutsche Nationalbibliografie; detailed bibliographic data are available on the Internet at <http://dnb.d-nb.de>. © 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Boschstr. 12, 69469 Weinheim, Germany All rights reserved (including those of translation into other languages). No part of this book may be reproduced in any form – by photoprinting, microfilm, or any other means – nor transmitted or translated into a machine language without written permission from the publishers. Registered names, trademarks, etc. used in this book, even when not specifically marked as such, are not to be considered unprotected by law. Print ISBN: 978-3-527-34398-0 ePDF ISBN: 978-3-527-81243-1 -

Biodegradable – Environmentally Aware Lubricants
No.118 page 1 LubePUBLISHED BY LUBE: THE EUROPEAN-- TechLUBRICANTS INDUSTRY MAGAZINE Biobased – Biodegradable – Environmentally aware lubricants Dr. Lou A. Honary, President, Environmental Lubricants Manufacturing, Inc. Historical Summary oleic sunflower oils as base oils. In the early 1990s, The interest in biobased lubricants and particularly the giant US agricultural equipment manufacturer greases are on the rise. Interestingly, the original Deere and Company introduced a Universal Tractor introduction of environment friendly lubricants began Transmission Hydraulic Fluid called Bio-Hy-Gard in Europe during the early 1980s. US researchers which had the research cooperation of The Lubrizol and lubricants experts followed Europe’s lead and Corporation’s additive technology. It was specifically the 1990s saw a huge developmental activity in designed to accommodate prevailing mandates in the the United States. Companies like The Lubrizol Black Forest areas in Germany. Caterpillar too later Corporation invested significant amount of resources introduced, a hydraulic fluid called Bio-Hydo. to develop additive packages for vegetable oil based hydraulic oils and focused on high oleic and ultra-high Introduction In 1991, this author founded a biobased research center at the University of Northern Iowa with support from the Iowa Soybean Promotion Board (ISPB) and the US Department of Agriculture among many other funding agencies. In 1997 a soybean oil-based version of Bio-Hy-Gard was introduced as a soybean oil-based universal tractor transmission hydraulic fluid with funding support from ISPB. This product has been under the ELM brand (Figure 1). Europe’s interest peaked again during the current century after research and developmental activities in the US had blossomed into a growing business. -

Edible Seeds
List of edible seeds This list of edible seeds includes seeds that are directly 1 Cereals foodstuffs, rather than yielding derived products. See also: Category:Cereals True cereals are the seeds of certain species of grass. Quinoa, a pseudocereal Maize A variety of species can provide edible seeds. Of the six major plant parts, seeds are the dominant source of human calories and protein.[1] The other five major plant parts are roots, stems, leaves, flowers, and fruits. Most ed- ible seeds are angiosperms, but a few are gymnosperms. The most important global seed food source, by weight, is cereals, followed by legumes, and nuts.[2] The list is divided into the following categories: • Cereals (or grains) are grass-like crops that are har- vested for their dry seeds. These seeds are often ground to make flour. Cereals provide almost half of all calories consumed in the world.[3] Botanically, true cereals are members of the Poaceae, the true grass family. A mixture of rices, including brown, white, red indica and wild rice (Zizania species) • Pseudocereals are cereal crops that are not Maize, wheat, and rice account for about half of the grasses. calories consumed by people every year.[3] Grains can be ground into flour for bread, cake, noodles, and other • Legumes including beans and other protein-rich food products. They can also be boiled or steamed, ei- soft seeds. ther whole or ground, and eaten as is. Many cereals are present or past staple foods, providing a large fraction of the calories in the places that they are eaten. -

Preparation and Characterization of Biodiesel from Melon Seed Oil and Tigernut Tuber Oil
University of Nigeria Research Publications SURMA, Nguamo Author Author PG/M.Sc/05/39981 Preparation and Characterization of Biodiesel From Title Melon Seed Oil and Tigernut Tuber Oil Physical Sciences Faculty Faculty Pure and Industrial Chemistry Department Department Date January, 2008 Signature Signature PREPARATION AND CHARACTERIZATION OF BIODIESEL FROM MELON SEED OIL AND TIGERNUT TUBER OIL SURMA, NGUAMO PGIM.Sc/05/39981 DEPARTMENT OF PURE AND INDUSTRIAL CHEMISTRY UNIVERSITY OF NIGERIA, NSUKKA JANUARY, 2008 PREPARATION AND CHARACTERIZATION OF BIODIESEL FROM MELON SEED OIL AND TIGERNUT TUBER OIL SURMA, NGUAMO PG/M.Sc/05/39981 A PROJECT SUBMITTED TO THE DEPARTMENT OF PURE AND INDUSTRIAL CHEMISTRY UNIVERSITY OF NIGERIA, NSUKKA JANUARY, 2008 CERTIFICATION SURMA, NGUAMO, a postgraduate student in the department of Pure and Industrial Chemistry with registration number PG/M.Sc/05/39981 has satisfactorily completed the requirements for course and research work for the degree of M.Sc. in Industrial Chemistry. The work embodied in this project work is original and has not been submitted in part or in full for any diploma or degree of this or any other university. PROF. C.A. NWADINIGWE DR. C.O.B. OKOYE (Supervisor) (Head of Department) DEDICATION This project work is dedicated to God Almighty for His guidance and protection and to the memory of my late mum Mrs. Esther Rumun Surma and also my dad Mr Robert Ityover Surma for the countless sacrifices made for my sake. ACKNOWLEDGEMENTS My utmost gratitude goes to God Almighty for His love, mercy, guidance and protection given to me to this stage of my life. -
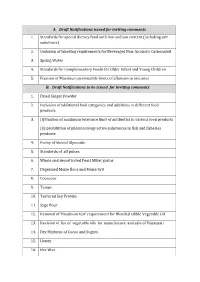
A. Draft Notifications Issued for Inviting Comments 1. Standards for Special Dietary Food with Low-Sodium Content (Including Salt Substitute)
A. Draft Notifications issued for inviting comments 1. Standards for special dietary food with low-sodium content (including salt substitute) 2. Omission of labelling requirements for Beverages Non-Alcoholic Carbonated 3. Spring Water 4. Standards for Complementary Foods for Older Infant and Young Children 5. Fixation of Maximum permissible limits of aflatoxin in arecanut B. Draft Notifications to be issued for inviting comments 1. Dried Ginger Powder 2. Inclusion of additional food categories and additives in different food products 3. (i)Fixation of maximum tolerance limit of antibiotics in various food products (ii) prohibition of pharmacology active substances in fish and fisheries products 4. Purity of Steviol Glyocside 5. Standards of all pulses 6. Whole and decorticated Pearl Millet grains 7. Degermed Maize flour and Maize Grit 8. Couscous 9. Tempe 10. Textured Soy Protein 11. Sago flour 12. Removal of ‘Boudouin test’ requirement for Blended edible Vegetable Oil 13. Revision of list of vegetable oils for manufacture and sale of Vanaspati 14. Dry Mixtures of Cocoa and Sugars 15. Honey 16. Bee Wax 17. Royal Jelly 18. Kachi Ghani Mustard Oil 19. Palm oil with regard to melting point 20. Vanaspati 21. Palm Stearin 22. Palm Kernel Olein 23. Palm Kernel Stearin 24. Superolein 25. Avocado Oil 26. Inclusion of Peroxide Value in standards of all vegetable oils 27. Meat and meat products (i) Fresh/Chilled/Frozen Pork (Pig meat) (ii) Fresh/Chilled/Frozen Beef (iii) Fresh/Chilled/Frozen Chevon (Goat meat) (iv) Fresh/Chilled/Frozen Mutton (Sheep meat) (v) Fresh/Chilled/Frozen Poultry meat (vi) Fresh eggs 28. -

Domesticated Plants List
List of domesticated plants • Loquat (Japanese medlar) • Common medlar • Pear • Quince 1.1.2 Citrus fruits Main article: Citrus • Citron This map shows the sites of domestication for a number of crops. Places where crops were initially domesticated are called centres • Grapefruit of origin • Lemon This is a list of plants that have been domesticated by • humans. Lime The list includes species or larger formal and informal • Orange botanical categories that include at least some domesti- • Pomelo cated individuals. To be considered domesticated, a population of plants must have their behavior, life cycle, or appearance signif- 1.1.3 Nut trees icantly altered as a result of being under humans control for multiple generations. (Please see the main article on Main article: Nut (fruit) domestication for more information.) Plants in this list are organized by the original or primary • Almond purpose for which they were domesticated. When a plant has more than one significant human use, it has been listed • Cashew in more than one category. • Chestnut • 1 Food and cooking Hazelnut • Macadamia 1.1 Fruit trees • Pecan Main article: List of Fruits • Pistachio • Walnut 1.1.1 Pomes 1.1.4 Other Main article: Pome • 103+ domesticated plant species in the Ama- zon, including sapodilla, calabash, tucuma, babacu, • Apple acai, wild pineapple, cocopalm, American-oil palm, Panama-hat palm, peach palm (Bactris gasipaes), • Asian pear ice-cream bean, 1 2 1 FOOD AND COOKING • Banana • Einkorn wheat (Triticum monococcum), now rarely grown. • Breadfruit • pasta -

Read Book in the Sweet Kitchen: the Definitive Bakers Companion Pdf
IN THE SWEET KITCHEN: THE DEFINITIVE BAKERS COMPANION PDF, EPUB, EBOOK Regan Daley | 692 pages | 01 Jul 2010 | ARTISAN | 9781579654276 | English | New York, United States In the Sweet Kitchen: The Definitive Bakers Companion PDF Book May 13, Rhi rated it it was amazing Shelves: eat-drink. Additional Product Features Dewey Edition. Just a moment while we sign you in to your Goodreads account. We have ratings, but no written reviews for this, yet. Amazon Kindle 0 editions. The recipes are delightful though. When Women Pray Hardcover T. Amazon Second Chance Pass it on, trade it in, give it a second life. Can I count this as a vegetable serving? You may also like. Return to Book Page. What makes a book so special and deserving that it gets chosen cookbook of the year? Friend Reviews. The recipes are great, with easy-to-follow instructions. List of vegetable oils. Move beyond carrots in baking to experience the larger world of sweet vegetables. The very last recipe in the book is a standard pie crust? I got tons of requests for the sugar cookie recipe after I gave them out as holiday presents. Error rating book. Bob rated it it was amazing Nov 20, Readers also enjoyed. These desserts are magic. A friend ordered a copy of this for herself and announced "Now I will have the secret baking powers you have too! Shelves: cookbooks. What makes a book so special and deserving that it gets chosen cookbook of the year? Other Editions 2. She currently lives in Toronto with her husband, three boys, and a food-phobic dog. -

(12) Patent Application Publication (10) Pub. No.: US 2007/0004811 A1 Bruner Et Al
US 20070004811A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0004811 A1 Bruner et al. (43) Pub. Date: Jan. 4, 2007 (54) POLYMERIZED OIL FOR USE ASA DUST Publication Classification CONTROL AGENT (51) Int. C. (75) Inventors: Michael C. Bruner, Naperville, IL C08, 5/20 (2006.01) (US): Selim M. Erhan, Riverside, IL C09K 3/22 (2006.01) (US); Brian M. Lenz, Des Plaines, IL U.S. Cl. ............................................. 521/27; 252/88.1 (US); Roger Scott Johnson, Snellville, (52) GA (US) Correspondence Address: BANNER & WTCOFF (57) ABSTRACT 1001 G STREET NW SUTE 11 OO WASHINGTON, DC 20001 (US) An aqueous dispersion of a polymerized (e.g., oxidized) oil, (73) Assignee: Georgia-Pacific Resins, Inc., Atlanta, preferably an oxidized vegetable oil, suitable for reducing GA (US) dusting in industrial processes, prepared by using, as the primary dispersing (emulsification) agent, a Sulfated or Appl. No.: 11/168,468 (21) sulfonated oil (also preferably a sulfated or sulfonated (22) Filed: Jun. 29, 2005 vegetable oil) to disperse the polymerized oil. US 2007/0004811 A1 Jan. 4, 2007 POLYMERIZED OIL FOR USE ASADUST carrier of a dust control agent and one of a water insoluble CONTROL AGENT elastomeric polymer, a pesticidal material or a biological control agent, respectively, for dust Suppression. An oil FIELD OF THE INVENTION (either mineral, vegetable or animal oil) is the main com 0001. The present invention relates to the use of an ponent for dust Suppression. aqueous dispersion of a polymerized (e.g., oxidized) oil, 0008 U.S. Pat. No. 6,355,083, U.S. -

(12) United States Patent (10) Patent No.: US 8.436,077 B2 El-Shoubary (45) Date of Patent: May 7, 2013
USOO8436077B2 (12) United States Patent (10) Patent No.: US 8.436,077 B2 El-Shoubary (45) Date of Patent: May 7, 2013 (54) LIPID-TREATED PARTICLES AND 2003/0O29.359 A1 2/2003 Marshall et al. 2005/0228112 A1* 10/2005 Takahashi et al. ............ 524,497 POLYMERS CONTAINING THE PARTICLES 2006/0047021 A1* 3/2006 Craig et al. ... ... 523,210 2006/01 11473 A1* 5/2006 Yuan et al. .................... 523,210 (75) Inventor: Modasser El-Shoubary, Crofton, MD (US) FOREIGN PATENT DOCUMENTS EP O535972 A1 4f1993 (73) Assignee: Cristal USA Inc., Hunt Valley, MD (US) EP 0628.303 A1 12, 1994 WO WO90-06103 6, 1990 (*) Notice: Subject to any disclaimer, the term of this WO WO93-11742 6, 1993 patent is extended or adjusted under 35 OTHER PUBLICATIONS U.S.C. 154(b) by 482 days. “File:Triglyceride-GeneralStructure.png; printed from Wikipedia website: http://en.wikipedia.org/wiki/File:Triglyceride (21) Appl. No.: 12/639,583 GeneralStructure.png; Oct. 28, 2009; 2 pages. 1-1. “Vegetable fats and oils”: printed from Wikipedia website: http://en. (22) Filed: Dec. 16, 2009 wikipedia.org/wiki/Vegetable oil; Oct. 28, 2009; 10 pages. "List of vegetable oils”: printed from Wikipedia website: http://en. (65) Prior Publication Data wikipedia.org/wiki/List of vegetable oils; Nov. 20, 2009; 16 US 2011/014.4251A1 Jun. 16, 2011 pageS. * cited by examiner (51) Int. Cl. CSK 9/00 (2006.01) Primary Examiner — Vickey Nerangis CSK 3/22 (2006.01) (74) Attorney, Agent, or Firm — Dunlap Codding, P.C.; (52) U.S. Cl. Tanzina Chowdhury USPC ........... -

International Journal of Advanced Scientific and Technical Research
International journal of advanced scientific and technical research Issue 4 volume 1, January-February 2014 Available online on http://www.rspublication.com/ijst/index.html ISSN 2249-9954 STUDIES ON THE CHEMICAL PROPERTIES OF SUNFLOWER, SESAME AND GROUNDNUT OILS *M.D.Sangale, 1A.S.Daptare * Head Department of Chemistry, A.A.College, Manchar, Dist-pune(M.S.) 1-Department of Chemistry, A.A.College, Manchar, Dist-pune(M.S.) Abstract: Natural oils and fats are triesters of glycerol with long chain carboxylic acid containing 12-20 carbon atoms these are known as triglycerides. Groundnut oil, sesame oil, sunflower oil, and mustered oil are rancidification is caused by two type of reactions namely hydrolysis and oxidation .antioxidants play an important role in the preservation of foods and other materials that deteriorate through oxidative changes. Sesame oil found to have a low peroxide value was added to sunflower and groundnut oil to check whether it subdues the peroxidation. Vitamin E as a synthetic antioxidant was also used for the same purpose. In recent years an increasing interest has developed in long chain ω-3 fatty acids such as EPA and DHA. So it has become a practice to consume ω-3 fatty acid rich oils in order to increase ω-3: ω-6 ratio in the diet. Several studies have indicated that consumption of ω-3 fatty acids can benefit persons with cardiovascular diseases and rheumatoid arthritis. However, the polyunsaturated fatty acids present in some edible oils because of their high degree of unsaturation have increased vulnerability to lipid peroxidation. To counteract this vulnerability, the practice of adding antioxidants to the oils has come in vogue. -

01. Sivasankaralingam V
STUDY ON BIODIESEL PRODUCED FROM INEDIBLE AND WASTE FEEDSTOCK IN A DIESEL ENGINE VEDHARAJ SIVASANKARALINGAM (B.Eng.), INDIA A THESIS SUBMITTED FOR THE DEGREE OF DOCTOR OF PHILOSOPHY DEPARTMENT OF MECHANICAL ENGINEERING NATIONAL UNIVERSITY OF SINGAPORE 2014 DECLARATION I hereby declare that the thesis is my original work and it has been written by me in its entirety. I have duly acknowledged all the sources of information which have been used in the thesis. This thesis has also not been submitted for any degree in any university previously. Vedharaj Sivasankaralingam 9 January 2014 ACKNOWLEDGEMENTS First and foremost, I wish to supplicate my reverence and obeisance to the living GODS - my parents, for showering all praises on me to accomplish my Ph.D. work. Undeniably, their support and motivation have helped me to perform better in my research and though I travelled in un-trodden path, I managed to achieve much because of their encouragement and affection. Professionally, I wish to thank my main supervisor, Prof. S.K. Chou, who gave his support and encouragement in this research work. As a matter of significance, he inculcated in me the habit to work independently and from his esteemed research career; I got to learn a lot. I would always remain grateful for his moral support and guidance throughout the course of my Ph.D. work. In the same note, I also thank my co-supervisor, Dr. Chua Kian Jon, Ernest, who provided his assistance to complete my Ph.D. work. Significantly, I was also under the auspice of Dr. Yang Wenming, who is an expert in the field of IC engines, biofuels and combustion and therefore, I would like to convey my profound sense of gratitude to him. -

Characterization of the Chemical Properties of Some Selected Refined Vegetable Oils Commonly Sold in Nigeria
British Journal of Applied Science & Technology 5(6): 538-546, 2015, Article no.BJAST.2015.052 ISSN: 2231-0843 SCIENCEDOMAIN international www.sciencedomain.org Characterization of the Chemical Properties of Some Selected Refined Vegetable Oils Commonly Sold in Nigeria Kelle Henrietta Ijeoma 1* and Udeozo Ifeoma Prisca 2 1Chemistry Unit, School of Science and Technology, National Open University of Nigeria, Victoria Island, Lagos, Nigeria. 2Department of Chemical Sciences, Tansian University, Umunya, Anambra State, Nigeria. Authors’ contributions This work was carried out in collaboration between all authors. Author KHI designed the study, performed the statistical analysis, wrote the protocol, and wrote the first draft of the manuscript and managed literature searches. Author UIP managed the analyses of the study and literature searches. All authors read and approved the final manuscript. Article Information DOI: 10.9734/BJAST/2015/12940 Editor(s): (1) Ming-Chih Shih, Department of Health and Nutrition Science, Chinese Culture University, Taiwan. Reviewers: (1) Anonymous, Sakarya University, Turkey. (2) Carlos Luna, Department of Organic Chemistry, University of Cordoba, Campus de Rabanales, Ed. Marie Curie,14014 Córdoba, Spain. Complete Peer review History: http://www.sciencedomain.org/review-history.php?iid=763&id=5&aid=6971 Received 24 th July 2014 th Original Research Article Accepted 5 September 2014 Published 15 th November 2014 ABSTRACT Aims: To ascertain the suitability for consumption of some selected refined vegetable oils