Lubricant Base
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polymerization of Safflower Oil in a Diesel Lubrication System by John Walter Olson a Thesis Submitted in Partial Fulfillment Of
Polymerization of safflower oil in a diesel lubrication system by John Walter Olson A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Chemical Engineering Montana State University © Copyright by John Walter Olson (1988) Abstract: Oxidative addition polymerization of lubrication oil contaminated with safflower oil diesel fuel was investigated in laboratory apparatus simulating conditions prevailing in a diesel engine crankcase. Primary research objectives were the identification and testing of key additives which might suppress problems of excessive viscosity rise and alkalinity decline of lubrication/safflower oil mixtures. Both commercial and base stock medium-speed locomotive lubrication oils were investigated with 5.0 weight percent safflower oil contamination. Oil mixtures were exposed to elevated temperature and were contacted by percolation with either oxygen or nitrogen to provide an oxidizing atmosphere and agitation or simply agitation. Copper, a known diesel engine wear metal, was introduced as a polymerization catalyst. Lubrication oils were evaluated with and without safflower oil and additives. Both commercial and base stock oils with no safflower oil were found to polymerize at a moderate baseline level. Amines were found to show little alkalinity maintenance efficacy while performance by an overbased calcium phenate was relatively good. Numerous antioxidants were tested for ability to disrupt the mechanisms leading to polymerization. Two widely-used antioxidants, zinc dialkyldithiophosphate and zinc diamyl-dithiocarbamate, were found to promote polymerization of oil mixtures in this research. This unexpected result is almost certainly a result of promotion catalysis by zinc. A metal deactivator N-N'-disalicylidene-1,2-propanediamine retarded viscosity rise, apparently by chelating wear metal catalysts. -

Improving Sexual Health: Vaginal Lubricants, Moisturizers, Dilators & Counseling
Improving Sexual Health: Vaginal Lubricants, Moisturizers, Dilators & Counseling Lubricants and moisturizers are effective in relieving pain during intercourse for many midlife women. If you have more severe vaginal dryness and related pain, or if lubricants and moisturizers don’t work well for you, see your health care provider as other options are available. How do vaginal lubricants work? Vaginal lubricants work by reducing the friction associated with thin, dry genital tissue. They come in liquid or gel form and are applied to the vagina and vulva right before intercourse (it can be helpful to apply them to the penis or any body part/instrument inserted into the vagina as well). Lubricants are absorbed into the skin, are immediate-acting, and provide temporary relief from vaginal dryness and related pain during intercourse. Lubricants are available either as water-based, silicone-based, or oil-based products. Water-based lubricants have the advantage of being non-staining and they wash off fairly easily. If you are concerned about staining your sheets, put down a large bath or beach towel on top of your sheets. Do not use oil-based lubricants (such as petroleum jelly and baby oil) because they can cause vaginal irritation. Additionally, oil-based lubricants may make condoms less effective. Some lubricants have glycerin and others are glycerin free. We recommend that you avoid glycerin, flavored lubricants, and warming/”tingling” lubricants. Silicone lubricants are often good options. For a list of lubricants that are available in drug and grocery stores, and online, see pages 4-5 of this handout. What are vaginal moisturizers? Vaginal moisturizers are products that are intended to ease vaginal dryness. -

Utilization of Waste Cooking Oil Via Recycling As Biofuel for Diesel Engines
recycling Article Utilization of Waste Cooking Oil via Recycling as Biofuel for Diesel Engines Hoi Nguyen Xa 1, Thanh Nguyen Viet 2, Khanh Nguyen Duc 2 and Vinh Nguyen Duy 3,* 1 University of Fire Fighting and Prevention, Hanoi 100000, Vietnam; [email protected] 2 School of Transportation and Engineering, Hanoi University of Science and Technology, Hanoi 100000, Vietnam; [email protected] (T.N.V.); [email protected] (K.N.D.) 3 Faculty of Vehicle and Energy Engineering, Phenikaa University, Hanoi 100000, Vietnam * Correspondence: [email protected] Received: 16 March 2020; Accepted: 2 June 2020; Published: 8 June 2020 Abstract: In this study, waste cooking oil (WCO) was used to successfully manufacture catalyst cracking biodiesel in the laboratory. This study aims to evaluate and compare the influence of waste cooking oil synthetic diesel (WCOSD) with that of commercial diesel (CD) fuel on an engine’s operating characteristics. The second goal of this study is to compare the engine performance and temperature characteristics of cooling water and lubricant oil under various engine operating conditions of a test engine fueled by waste cooking oil and CD. The results indicated that the engine torque of the engine running with WCOSD dropped from 1.9 Nm to 5.4 Nm at all speeds, and its brake specific fuel consumption (BSFC) dropped at almost every speed. Thus, the thermal brake efficiency (BTE) of the engine fueled by WCOSD was higher at all engine speeds. Also, the engine torque of the WCOSD-fueled engine was lower than the engine torque of the CD-fueled engine at all engine speeds. -

Cosmetic Creams Cosmetic Creams
Cosmetic Creams Cosmetic Creams Development, Manufacture and Marketing of Effective Skin Care Products Wilfried Rähse Author All books published by Wiley-VCH are carefully produced. Nevertheless, Dr. Wilfried Rähse authors, editors, and publisher do not Bahlenstr. 168 warrant the information contained in 40589 Düsseldorf these books, including this book, to Germany be free of errors. Readers are advised to keep in mind that statements, data, Cover Images: © keng88/Shutterstock, illustrations, procedural details or other © Arthur S. Aubry/Getty Images items may inadvertently be inaccurate. Library of Congress Card No.: applied for British Library Cataloguing-in-Publication Data A catalogue record for this book is available from the British Library. Bibliographic information published by the Deutsche Nationalbibliothek The Deutsche Nationalbibliothek lists this publication in the Deutsche Nationalbibliografie; detailed bibliographic data are available on the Internet at <http://dnb.d-nb.de>. © 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Boschstr. 12, 69469 Weinheim, Germany All rights reserved (including those of translation into other languages). No part of this book may be reproduced in any form – by photoprinting, microfilm, or any other means – nor transmitted or translated into a machine language without written permission from the publishers. Registered names, trademarks, etc. used in this book, even when not specifically marked as such, are not to be considered unprotected by law. Print ISBN: 978-3-527-34398-0 ePDF ISBN: 978-3-527-81243-1 -

Lubricants in Pharmaceutical Solid Dosage Forms
Lubricants 2014, 2, 21-43; doi:10.3390/lubricants2010021 OPEN ACCESS lubricants ISSN 2075-4442 www.mdpi.com/journal/lubricants Review Lubricants in Pharmaceutical Solid Dosage Forms Jinjiang Li * and Yongmei Wu Drug Product Science & Technology, Bristol-Myers Squibb Corporation, 1 Squibb Dr., New Brunswick, NJ 08903, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-732-227-6584; Fax: +1-732-227-3784. Received: 18 December 2013; in revised form: 21 January 2014 / Accepted: 24 January 2014 / Published: 25 February 2014 Abstract: Lubrication plays a key role in successful manufacturing of pharmaceutical solid dosage forms; lubricants are essential ingredients in robust formulations to achieve this. Although many failures in pharmaceutical manufacturing operations are caused by issues related to lubrication, in general, lubricants do not gain adequate attention in the development of pharmaceutical formulations. In this paper, the fundamental background on lubrication is introduced, in which the relationships between lubrication and friction/adhesion forces are discussed. Then, the application of lubrication in the development of pharmaceutical products and manufacturing processes is discussed with an emphasis on magnesium stearate. In particular, the effect of its hydration state (anhydrate, monohydrate, dihydrate, and trihydrate) and its powder characteristics on lubrication efficiency, as well as product and process performance is summarized. In addition, the impact of lubrication on the dynamics of compaction/compression processes and on the mechanical properties of compacts/tablets is presented. Furthermore, the online monitoring of magnesium stearate in a blending process is briefly mentioned. Finally, the chemical compatibility of active pharmaceutical ingredient (API) with magnesium stearate and its reactive impurities is reviewed with examples from the literature illustrating the various reaction mechanisms involved. -

Biodegradable – Environmentally Aware Lubricants
No.118 page 1 LubePUBLISHED BY LUBE: THE EUROPEAN-- TechLUBRICANTS INDUSTRY MAGAZINE Biobased – Biodegradable – Environmentally aware lubricants Dr. Lou A. Honary, President, Environmental Lubricants Manufacturing, Inc. Historical Summary oleic sunflower oils as base oils. In the early 1990s, The interest in biobased lubricants and particularly the giant US agricultural equipment manufacturer greases are on the rise. Interestingly, the original Deere and Company introduced a Universal Tractor introduction of environment friendly lubricants began Transmission Hydraulic Fluid called Bio-Hy-Gard in Europe during the early 1980s. US researchers which had the research cooperation of The Lubrizol and lubricants experts followed Europe’s lead and Corporation’s additive technology. It was specifically the 1990s saw a huge developmental activity in designed to accommodate prevailing mandates in the the United States. Companies like The Lubrizol Black Forest areas in Germany. Caterpillar too later Corporation invested significant amount of resources introduced, a hydraulic fluid called Bio-Hydo. to develop additive packages for vegetable oil based hydraulic oils and focused on high oleic and ultra-high Introduction In 1991, this author founded a biobased research center at the University of Northern Iowa with support from the Iowa Soybean Promotion Board (ISPB) and the US Department of Agriculture among many other funding agencies. In 1997 a soybean oil-based version of Bio-Hy-Gard was introduced as a soybean oil-based universal tractor transmission hydraulic fluid with funding support from ISPB. This product has been under the ELM brand (Figure 1). Europe’s interest peaked again during the current century after research and developmental activities in the US had blossomed into a growing business. -

Vegetable Oils As Lube Basestocks: a Review
African Journal of Biotechnology Vol. 12(9), pp. 880-891, 27 February, 2013 Available online at http://www.academicjournals.org/AJB DOI: 10.5897/AJB12.2823 ISSN 1684–5315 ©2013 Academic Journals Review Vegetable oils as lube basestocks: A review Anjana Srivastava1* and Preeti Sahai2 1Department of Chemistry, Amity Institute of Applied Sciences, Amity University, UP, India. 2Department of Science and Technology, Technological Development Board, India. Accepted 15 February, 2013 The depletion of the world’s crude oil reserve, increasing crude oil prices, and issues related to conservation have brought about renewed interest in the use of bio-based materials. Emphasis on the development of renewable, biodegradable, and environmentally friendly lubricants has resulted in the widespread use of natural oils and fats. Vegetable oils are promising candidates as base fluid for eco- friendly lubricants because of their excellent lubricity, biodegradability, viscosity-temperature characteristics and low volatility. In view of agriculture based Indian economy, there is a great potential of producing vegetable oil based lubricants, which has ecological compatibility in addition to technical performance. However, suitability of the vegetable oils for a specific application either needs chemical modification or may be used as it is with additive blending route in order to get basestocks as per specifications for a particular end use application. Key words: Biolubricants, vegetable oils, biodegradable, renewable. INTRODUCTION In recent years, deliberate and accidental lubricant losses products because of their environment-friendly and to the environment by means including evaporation, nontoxic nature. Synthetic lubricant base oils are also leakages, and spills have lead to major concerns available and offer improved stability and performance regarding pollution and environmental health. -

Vegetable Oils As Lubricant Base
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 ISSN 2229-5518 708 9HJHWDEOHRLOVDVOXEULFDQWEDVHRLO$5HYLHZ RANI.S, 7$581060/-2<.35$%+$.$5$11$,5 'HSDUWPHQWRI0HFKDQLFDO(QJLQHHULQJ 1DWLRQDO,QVWLWXWHRI7HFKQRORJ\&DOLFXW&DOLFXW Abstract— The demand for industrial lubricants is increasing were based on vegetable oils. In the US around 8,250,000 day by day. Due to the industrial revolution most of the Asian tons/year were consumed in 2002 among which 25,000 countries becomes the manufacturing centers for the world. Lot tons/year were based on vegetables [6]. of used lubricants is thrown to the environment which may damage the environment. In this scenario industry started searching for environmental friendly, renewable and less toxic lubricants . The biolubricant formulation is one of the upcoming research areas in tribology. In this paper the lubricant properties of vegetable oils have been discussed with the available literature. Keywords— environmental friendly; renewable; Biolubricant formulation I. INTRODUCTION Mineral oil and synthetic oil from petrochemical are mainly used as base oil in present lubricant industry. The Fig. 1. Worldwide lubrication consumption in 2004 [4] petroleum resources are diminishing rapidly and the rate of production from older domestic oil fields and decrease in the rate of finding new reservesIJSER are one of the main problems of The renewable and biodegradable vegetable oils have been the 21st century. Petroleum products are poorly degradable [1] used various industrial applications like Chain saw bar and cause severe environmental hazards when released [2] lubricants [7], engine lubricants [8,9], drilling lubricants [10], .The spilling of mineral oil causes negative changes in the metal cutting fluid [11,12], hydraulic fluids [13,14] and sand which affects the agricultural growth a lot [3]. -

Edible Seeds
List of edible seeds This list of edible seeds includes seeds that are directly 1 Cereals foodstuffs, rather than yielding derived products. See also: Category:Cereals True cereals are the seeds of certain species of grass. Quinoa, a pseudocereal Maize A variety of species can provide edible seeds. Of the six major plant parts, seeds are the dominant source of human calories and protein.[1] The other five major plant parts are roots, stems, leaves, flowers, and fruits. Most ed- ible seeds are angiosperms, but a few are gymnosperms. The most important global seed food source, by weight, is cereals, followed by legumes, and nuts.[2] The list is divided into the following categories: • Cereals (or grains) are grass-like crops that are har- vested for their dry seeds. These seeds are often ground to make flour. Cereals provide almost half of all calories consumed in the world.[3] Botanically, true cereals are members of the Poaceae, the true grass family. A mixture of rices, including brown, white, red indica and wild rice (Zizania species) • Pseudocereals are cereal crops that are not Maize, wheat, and rice account for about half of the grasses. calories consumed by people every year.[3] Grains can be ground into flour for bread, cake, noodles, and other • Legumes including beans and other protein-rich food products. They can also be boiled or steamed, ei- soft seeds. ther whole or ground, and eaten as is. Many cereals are present or past staple foods, providing a large fraction of the calories in the places that they are eaten. -

Glossary of Petrochemical Terms
GLOSSARY OF PETROCHEMICAL TERMS Acid Number: A measure of the amount of potassium hydroxide (KOH) needed to neutralize all or part of the acidity of a petroleum product. Also specified as neutralization number (NN) or value (NV) and total acid number (TAN) Additive: A chemical substance which, when blended with a petroleum product, has the effect of improving one or more of its properties or performance characteristics. Aliphatic Hydrocarbon: Hydrocarbons in which the carbon atoms are arranged in open chains which may be straight or branched. Aniline Point: The minimum temperature for complete miscibility of equal volumes of aniline and the sample under test. Products with high aromatic or naphthenic contents have lower aniline points than products with high paraffinic content. Anti-knock: Resistance of a gasoline (petrol) to detonation in a combustion chamber. API Gravity: A special function of relative density represented by: API Gravity, degrees = 141.5/rel.density at 15.6°C – 131.5 API Service Classification: A system of letter designations agreed by API, SAE and ASTM to define broad classes of engine service. Also used for service classification of automotive gear lubricants. Aromatic: A hydrocarbon derived from, or characterized by, the presence of a benzene ring, or a polymeric (multiple) ring structure. Ash: Non-combustible residue of lubricating oil or fuel,; lubricating oil detergent additives containing metallic derivatives are a common source of ash (see also sulphated ash). Bactericide: An additive to inhibit bacterial growth in aqueous component or phase of fluids, preventing bacterial degradation of the fluid and the resulting foul odours. Base Number: The amount of acid required to neutralize all or part of a lubricant’s basicity, expressed as potassium hydroxide (KOH) equivalents. -

Preparation and Characterization of Biodiesel from Melon Seed Oil and Tigernut Tuber Oil
University of Nigeria Research Publications SURMA, Nguamo Author Author PG/M.Sc/05/39981 Preparation and Characterization of Biodiesel From Title Melon Seed Oil and Tigernut Tuber Oil Physical Sciences Faculty Faculty Pure and Industrial Chemistry Department Department Date January, 2008 Signature Signature PREPARATION AND CHARACTERIZATION OF BIODIESEL FROM MELON SEED OIL AND TIGERNUT TUBER OIL SURMA, NGUAMO PGIM.Sc/05/39981 DEPARTMENT OF PURE AND INDUSTRIAL CHEMISTRY UNIVERSITY OF NIGERIA, NSUKKA JANUARY, 2008 PREPARATION AND CHARACTERIZATION OF BIODIESEL FROM MELON SEED OIL AND TIGERNUT TUBER OIL SURMA, NGUAMO PG/M.Sc/05/39981 A PROJECT SUBMITTED TO THE DEPARTMENT OF PURE AND INDUSTRIAL CHEMISTRY UNIVERSITY OF NIGERIA, NSUKKA JANUARY, 2008 CERTIFICATION SURMA, NGUAMO, a postgraduate student in the department of Pure and Industrial Chemistry with registration number PG/M.Sc/05/39981 has satisfactorily completed the requirements for course and research work for the degree of M.Sc. in Industrial Chemistry. The work embodied in this project work is original and has not been submitted in part or in full for any diploma or degree of this or any other university. PROF. C.A. NWADINIGWE DR. C.O.B. OKOYE (Supervisor) (Head of Department) DEDICATION This project work is dedicated to God Almighty for His guidance and protection and to the memory of my late mum Mrs. Esther Rumun Surma and also my dad Mr Robert Ityover Surma for the countless sacrifices made for my sake. ACKNOWLEDGEMENTS My utmost gratitude goes to God Almighty for His love, mercy, guidance and protection given to me to this stage of my life. -
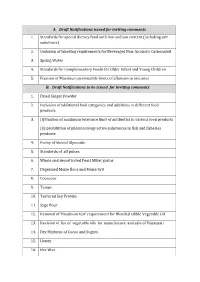
A. Draft Notifications Issued for Inviting Comments 1. Standards for Special Dietary Food with Low-Sodium Content (Including Salt Substitute)
A. Draft Notifications issued for inviting comments 1. Standards for special dietary food with low-sodium content (including salt substitute) 2. Omission of labelling requirements for Beverages Non-Alcoholic Carbonated 3. Spring Water 4. Standards for Complementary Foods for Older Infant and Young Children 5. Fixation of Maximum permissible limits of aflatoxin in arecanut B. Draft Notifications to be issued for inviting comments 1. Dried Ginger Powder 2. Inclusion of additional food categories and additives in different food products 3. (i)Fixation of maximum tolerance limit of antibiotics in various food products (ii) prohibition of pharmacology active substances in fish and fisheries products 4. Purity of Steviol Glyocside 5. Standards of all pulses 6. Whole and decorticated Pearl Millet grains 7. Degermed Maize flour and Maize Grit 8. Couscous 9. Tempe 10. Textured Soy Protein 11. Sago flour 12. Removal of ‘Boudouin test’ requirement for Blended edible Vegetable Oil 13. Revision of list of vegetable oils for manufacture and sale of Vanaspati 14. Dry Mixtures of Cocoa and Sugars 15. Honey 16. Bee Wax 17. Royal Jelly 18. Kachi Ghani Mustard Oil 19. Palm oil with regard to melting point 20. Vanaspati 21. Palm Stearin 22. Palm Kernel Olein 23. Palm Kernel Stearin 24. Superolein 25. Avocado Oil 26. Inclusion of Peroxide Value in standards of all vegetable oils 27. Meat and meat products (i) Fresh/Chilled/Frozen Pork (Pig meat) (ii) Fresh/Chilled/Frozen Beef (iii) Fresh/Chilled/Frozen Chevon (Goat meat) (iv) Fresh/Chilled/Frozen Mutton (Sheep meat) (v) Fresh/Chilled/Frozen Poultry meat (vi) Fresh eggs 28.