Cosmetic Creams Cosmetic Creams
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Essential Oils
-Essential Oils- Essential Oils エッセンシャルオイル An essential oil is a natural, 100% pure oil extracted by distillation or another method from various sources such as aromatic substances produced in the flowers, buds, leaves, peel, bark, roots, or seeds of plants, or organs of certain animals that secrete aromatic components. Essential oils are used in aromatherapy, a natural remedy to cure psychological stress and poor physical conditions, and are also used as food flavorings to be added to beverages, confectionery and other processed foods, and as cosmetic fragrances for perfume products or toiletries. Essential oils have long been used for scenting purposes such as food flavorings and cosmetic fragrances, but the glowing popularity of aromatherapy in recent years has resulted in increased uses of essential oils for aromatherapy. Scope of coverage Item Definition HS Code Citrus essential oils Of orange 3301.12.000 Of lemon 3301.13.000 Other (bergamot, other) 3301.19 Non-citrus essential oils Other peppermint (Mentha piperita) 3301.24.000 Of other mints 3301.25 Other 3301.29. Resinoid - 3301.30.000 Other - 3301.90.000 Note: Resinoid refers to liquid, semisolid, or solid substances extracted from plant resins or other sources with the use of a hydrocarbon solvent. 1. Points To Note in Exports to and Sales in Japan (1) Import Regulation and Procedures Importing essential oils may be subject to regulations under the Foreign Exchange and Foreign Trade Act, based on the CITES[Convention on International Trade in Endangered Species of Wild Fauna and Flora]. Different procedures may also be required according to uses of essential oils. -

Bay Oil (Pimenta Racemosa)
the world of pure essential oils Home Bay essential oil information Contact us Shopping security West Indian Bay essential oil is extracted from the Laurus nobilis tree, of the Lauraceae family and is also known as sweet bay, laurel and Mediterranean bay. Orders in U.S.A. and World Essential oils Other oils On this page African oils Carrier oils Oil properties Pre-blended oils Origin of bay oil Caucheteux fragrancing Extraction Worldwide shipping Chemical composition ,Ł,Ľ.... converter Precautions Therapeutic properties Uses Summary Buying bay oil from us Orders in South Africa (Suid Afrika) Essential oils Other oils African oils Go to shopping cart at bottom of page Carrier oils Pre-blended oils Aflewering | Delivery This warming oil is often used in aromatherapy since it is a good antiseptic for the respiratory system, perks up the digestive system, settles stomach pain and expels wind, while promoting confidence, courage and insight. Topically, it is most often used to combat hair loss and to improve INFORMATION the health of the scalp in general. Essential oil index Oil properties Ways to use oils The scent of bay oil is sweet, fresh and spicy and the oil is deep yellow in color, being of medium to Recipes watery viscosity. Dilution rates Safety Origin of bay oil Glossary of terms Treating ailments This sturdy evergreen tree is a native of West Indies, Venezuela and the Guianas. Nowadays the oil Uses is obtained mostly from Morocco and Spain. The bay tree grows to about 10 meters (30 feet), has Oil extraction long aromatic lance-shaped leaves, small white-yellow flowers and black berries. -

CLINICAL AROMATHERAPY 2E ISBN 0-443-07236-1 Copyright 2003, Elsevier Science
CHURCHILL LIVINGSTONE An Imprint of Elsevier Science Publishing Manager: Inta Izols Development Editor: Karen Gilmour Project Manager: Peggy Fagen Design Manager: Mark Bernard Design: Sheilah Barrett Design CLINICAL AROMATHERAPY 2e ISBN 0-443-07236-1 Copyright 2003, Elsevier Science. All rights reserved. No part of this publication may be reproduced in any form or by any means, elec- tronic or mechanical, including photocopy, recording, or any information storage and retrieval system, without permission of the publisher (Churchill Livingstone, The Curtis Center, Independence Square West, Philadelphia, PA 19106). Churchill Livingstone and the sailboat design are registered trademarks. NOTICE Complementary and alternative medicine is an ever-changing field. Standard safety precautions must be followed, but as new research and clinical experience broaden our knowledge, change in treatment and drug therapy may become necessary as appropriate. Readers are advised to check the most cur- rent product information provided by the manufacturer of each drug to be administered to verify the recommended dose, the method and duration of administration, and contraindications. It is the re- sponsibility of the licensed prescriber, relying on experience and knowledge of the patient, to deter- mine dosages and the best treatment for each individual patient. Neither the publisher nor the editors assume any liability for any injury and/or damage to persons or property arising from this publication. Library of Congress Cataloging in Publication Data Buckle, Jane, RGN, MA, BPhil, Cert Ed, MISPA, MIScB. Clinical aromatherapy / Jane Buckle.—2nd ed. p. ; cm. Rev. ed. of: Clinical aromatherapy in nursing / Jane Buckle. c1997. Includes bibliographical references and index. ISBN 0-443-07236-1 1. -

Biodegradable – Environmentally Aware Lubricants
No.118 page 1 LubePUBLISHED BY LUBE: THE EUROPEAN-- TechLUBRICANTS INDUSTRY MAGAZINE Biobased – Biodegradable – Environmentally aware lubricants Dr. Lou A. Honary, President, Environmental Lubricants Manufacturing, Inc. Historical Summary oleic sunflower oils as base oils. In the early 1990s, The interest in biobased lubricants and particularly the giant US agricultural equipment manufacturer greases are on the rise. Interestingly, the original Deere and Company introduced a Universal Tractor introduction of environment friendly lubricants began Transmission Hydraulic Fluid called Bio-Hy-Gard in Europe during the early 1980s. US researchers which had the research cooperation of The Lubrizol and lubricants experts followed Europe’s lead and Corporation’s additive technology. It was specifically the 1990s saw a huge developmental activity in designed to accommodate prevailing mandates in the the United States. Companies like The Lubrizol Black Forest areas in Germany. Caterpillar too later Corporation invested significant amount of resources introduced, a hydraulic fluid called Bio-Hydo. to develop additive packages for vegetable oil based hydraulic oils and focused on high oleic and ultra-high Introduction In 1991, this author founded a biobased research center at the University of Northern Iowa with support from the Iowa Soybean Promotion Board (ISPB) and the US Department of Agriculture among many other funding agencies. In 1997 a soybean oil-based version of Bio-Hy-Gard was introduced as a soybean oil-based universal tractor transmission hydraulic fluid with funding support from ISPB. This product has been under the ELM brand (Figure 1). Europe’s interest peaked again during the current century after research and developmental activities in the US had blossomed into a growing business. -

Aveda Aromaology I: Essence of Aveda Leader Guide Introduction
Aveda Aromaology I: Essence of Aveda Leader Guide Introduction: Aveda Aromaology 1 Essence of Aveda Welcome the participants to the class. Introduce yourself and your background. Have the participants introduce themselves mentioning name, salon/spa, role (hairdresser, spa therapist, owner, guest services, etc) their background in aromatherapy/ aromaology and any expectations they have for the class today. Cover any logistics with food, restrooms, etc. Review the Aveda Mission: OUR MISSION AT AVEDA IS TO CARE FOR THE WORLD WE LIVE IN FROM THE PRODCUTS WE MAKE TO THE WAYS IN WHICH WE GIVE BACK TO SOCIETY. AT AVEDA WE STRIVE TO SET AN EXAMPLE FOR ENVIRONMENTAL LEADERSHIP AND RESPONSIBILITY NOTJUST IN THE WORLD OF BEAUTY BUT AROUND THE WORLD. Aromaology is the heart of Aveda. It is one of the founding principles of Aveda and is important part of our mission of caring for the world through making products with organic sustainable ingredients On the power point show a few images of keystones as well as cars. Have the group look at the images and write down their thoughts when they see the images. Have the group share their ideas: Look for beauty, form, emotions, etc. In addition lead a discussion with the group about the function of keystones. Without the keystone, the entire structure would fall apart. It is a weight bearing stone that serves the function of stability in design. Often times when we see nice cars, we think of the comfort and features of the car. But the car’s function is to get us from point A to B. -

Edible Seeds
List of edible seeds This list of edible seeds includes seeds that are directly 1 Cereals foodstuffs, rather than yielding derived products. See also: Category:Cereals True cereals are the seeds of certain species of grass. Quinoa, a pseudocereal Maize A variety of species can provide edible seeds. Of the six major plant parts, seeds are the dominant source of human calories and protein.[1] The other five major plant parts are roots, stems, leaves, flowers, and fruits. Most ed- ible seeds are angiosperms, but a few are gymnosperms. The most important global seed food source, by weight, is cereals, followed by legumes, and nuts.[2] The list is divided into the following categories: • Cereals (or grains) are grass-like crops that are har- vested for their dry seeds. These seeds are often ground to make flour. Cereals provide almost half of all calories consumed in the world.[3] Botanically, true cereals are members of the Poaceae, the true grass family. A mixture of rices, including brown, white, red indica and wild rice (Zizania species) • Pseudocereals are cereal crops that are not Maize, wheat, and rice account for about half of the grasses. calories consumed by people every year.[3] Grains can be ground into flour for bread, cake, noodles, and other • Legumes including beans and other protein-rich food products. They can also be boiled or steamed, ei- soft seeds. ther whole or ground, and eaten as is. Many cereals are present or past staple foods, providing a large fraction of the calories in the places that they are eaten. -

Chemical Composition and Biological Activities of Essential Oils of Essential and Biological Activities Composition Chemical
Chemical Composition Activities and Biological Essential of Oils Chemical • Edoardo Napoli Marco and Maura Di Vito Composition and Biological Activities of Essential Oils Edited by Edoardo Marco Napoli and Maura Di Vito Printed Edition of the Special Issue Published in Antibiotics www.mdpi.com/journal/antibiotics Chemical Composition and Biological Activities of Essential Oils Chemical Composition and Biological Activities of Essential Oils Editors Edoardo Marco Napoli Maura Di Vito MDPI • Basel • Beijing • Wuhan • Barcelona • Belgrade • Manchester • Tokyo • Cluj • Tianjin Editors Edoardo Marco Napoli Maura Di Vito Institute of Biomolecular University of Bologna Chemistry Italy National Research Council Universit`a Cattolica del Sacro Cuore ICB-CNR Italy Italy Editorial Office MDPI St. Alban-Anlage 66 4052 Basel, Switzerland This is a reprint of articles from the Special Issue published online in the open access journal Antibiotics (ISSN 2079-6382) (available at: https://www.mdpi.com/journal/antibiotics/special issues/Chemical Composition). For citation purposes, cite each article independently as indicated on the article page online and as indicated below: LastName, A.A.; LastName, B.B.; LastName, C.C. Article Title. Journal Name Year, Volume Number, Page Range. ISBN 978-3-0365-1250-1 (Hbk) ISBN 978-3-0365-1251-8 (PDF) Cover image courtesy of Edoardo Napoli and Maria Grazia Bellardi. © 2021 by the authors. Articles in this book are Open Access and distributed under the Creative Commons Attribution (CC BY) license, which allows users to download, copy and build upon published articles, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. -

ESSENTIAL SKIN CARE WHAT SETS Dōterra® ESSENTIAL SKIN CARE APART?
® ESSENTIAL SKIN CARE WHAT SETS dōTERRA® ESSENTIAL SKIN CARE APART? By integrating CPTG Certified Pure Tested Grade® essential oils with cutting-edge technologies, dōTERRA Essential Skin Care products provide scientifically proven ingredients and botanicals resulting in radiant, youthful-looking skin. ESSENTIAL SKIN CARE COLLECTION dōTERRA Essential Skin Care is a family of skin care products designed to meet all your skin care needs, helping keep your skin feeling and looking young and healthy. FACIAL CLEANSER essential oils of TEA TREE and PEPPERMINT dōTERRA® Facial Cleanser combines CPTG® essential oils of Tea Tree and Peppermint, known for their ability to purify and tone skin, with naturally sourced cleansers of Yucca Root extract and Soapbark extract, which gently wash away impurities leaving skin looking clean, fresh, and smooth. • Vitamin E supports healthy-looking skin • Macadamia seed oil conditions the skin • Yucca Root extract and Soapbark extract are rich in saponins, cleansers that clarify and soothe skin DIRECTIONS FOR USE: Dispense cleanser into palm of hand. Apply to dry face and neck in a circular motion. Rinse with warm water and towel dry. Avoid contact with eyes. 60203376 118 mL $28.33 CAD retail $21.25 CAD wholesale 17.5 PV INGREDIENTS: Aqua, Sodium Cocoyl Isethionate, Stearic Acid, Glycerin, Glyceryl Stearate SE, Cetearyl Alcohol, Benzyl Alcohol, Xanthan Gum, Macadamia Ternifolia Seed Oil, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Mentha Piperita (Peppermint) Oil, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Chlorphenesin, Sodium Benzoate, Sodium Phytate, Dehydroacetic Acid, Sodium PCA, Tocopherol, Citric Acid, Potassium Hydroxide, Quillaja Saponaria Bark Extract, Yucca Filamentosa Root Extract INVIGORATING SCRUB essential oils of GRAPEFRUIT and PEPPERMINT CPTG® essential oils of Grapefruit and Peppermint make exfoliating a refreshing aromatic experience while Jojoba esters polish your skin. -

Preparation and Characterization of Biodiesel from Melon Seed Oil and Tigernut Tuber Oil
University of Nigeria Research Publications SURMA, Nguamo Author Author PG/M.Sc/05/39981 Preparation and Characterization of Biodiesel From Title Melon Seed Oil and Tigernut Tuber Oil Physical Sciences Faculty Faculty Pure and Industrial Chemistry Department Department Date January, 2008 Signature Signature PREPARATION AND CHARACTERIZATION OF BIODIESEL FROM MELON SEED OIL AND TIGERNUT TUBER OIL SURMA, NGUAMO PGIM.Sc/05/39981 DEPARTMENT OF PURE AND INDUSTRIAL CHEMISTRY UNIVERSITY OF NIGERIA, NSUKKA JANUARY, 2008 PREPARATION AND CHARACTERIZATION OF BIODIESEL FROM MELON SEED OIL AND TIGERNUT TUBER OIL SURMA, NGUAMO PG/M.Sc/05/39981 A PROJECT SUBMITTED TO THE DEPARTMENT OF PURE AND INDUSTRIAL CHEMISTRY UNIVERSITY OF NIGERIA, NSUKKA JANUARY, 2008 CERTIFICATION SURMA, NGUAMO, a postgraduate student in the department of Pure and Industrial Chemistry with registration number PG/M.Sc/05/39981 has satisfactorily completed the requirements for course and research work for the degree of M.Sc. in Industrial Chemistry. The work embodied in this project work is original and has not been submitted in part or in full for any diploma or degree of this or any other university. PROF. C.A. NWADINIGWE DR. C.O.B. OKOYE (Supervisor) (Head of Department) DEDICATION This project work is dedicated to God Almighty for His guidance and protection and to the memory of my late mum Mrs. Esther Rumun Surma and also my dad Mr Robert Ityover Surma for the countless sacrifices made for my sake. ACKNOWLEDGEMENTS My utmost gratitude goes to God Almighty for His love, mercy, guidance and protection given to me to this stage of my life. -
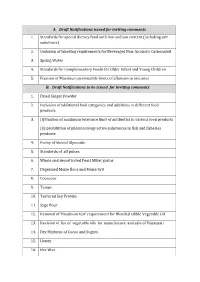
A. Draft Notifications Issued for Inviting Comments 1. Standards for Special Dietary Food with Low-Sodium Content (Including Salt Substitute)
A. Draft Notifications issued for inviting comments 1. Standards for special dietary food with low-sodium content (including salt substitute) 2. Omission of labelling requirements for Beverages Non-Alcoholic Carbonated 3. Spring Water 4. Standards for Complementary Foods for Older Infant and Young Children 5. Fixation of Maximum permissible limits of aflatoxin in arecanut B. Draft Notifications to be issued for inviting comments 1. Dried Ginger Powder 2. Inclusion of additional food categories and additives in different food products 3. (i)Fixation of maximum tolerance limit of antibiotics in various food products (ii) prohibition of pharmacology active substances in fish and fisheries products 4. Purity of Steviol Glyocside 5. Standards of all pulses 6. Whole and decorticated Pearl Millet grains 7. Degermed Maize flour and Maize Grit 8. Couscous 9. Tempe 10. Textured Soy Protein 11. Sago flour 12. Removal of ‘Boudouin test’ requirement for Blended edible Vegetable Oil 13. Revision of list of vegetable oils for manufacture and sale of Vanaspati 14. Dry Mixtures of Cocoa and Sugars 15. Honey 16. Bee Wax 17. Royal Jelly 18. Kachi Ghani Mustard Oil 19. Palm oil with regard to melting point 20. Vanaspati 21. Palm Stearin 22. Palm Kernel Olein 23. Palm Kernel Stearin 24. Superolein 25. Avocado Oil 26. Inclusion of Peroxide Value in standards of all vegetable oils 27. Meat and meat products (i) Fresh/Chilled/Frozen Pork (Pig meat) (ii) Fresh/Chilled/Frozen Beef (iii) Fresh/Chilled/Frozen Chevon (Goat meat) (iv) Fresh/Chilled/Frozen Mutton (Sheep meat) (v) Fresh/Chilled/Frozen Poultry meat (vi) Fresh eggs 28. -

Domesticated Plants List
List of domesticated plants • Loquat (Japanese medlar) • Common medlar • Pear • Quince 1.1.2 Citrus fruits Main article: Citrus • Citron This map shows the sites of domestication for a number of crops. Places where crops were initially domesticated are called centres • Grapefruit of origin • Lemon This is a list of plants that have been domesticated by • humans. Lime The list includes species or larger formal and informal • Orange botanical categories that include at least some domesti- • Pomelo cated individuals. To be considered domesticated, a population of plants must have their behavior, life cycle, or appearance signif- 1.1.3 Nut trees icantly altered as a result of being under humans control for multiple generations. (Please see the main article on Main article: Nut (fruit) domestication for more information.) Plants in this list are organized by the original or primary • Almond purpose for which they were domesticated. When a plant has more than one significant human use, it has been listed • Cashew in more than one category. • Chestnut • 1 Food and cooking Hazelnut • Macadamia 1.1 Fruit trees • Pecan Main article: List of Fruits • Pistachio • Walnut 1.1.1 Pomes 1.1.4 Other Main article: Pome • 103+ domesticated plant species in the Ama- zon, including sapodilla, calabash, tucuma, babacu, • Apple acai, wild pineapple, cocopalm, American-oil palm, Panama-hat palm, peach palm (Bactris gasipaes), • Asian pear ice-cream bean, 1 2 1 FOOD AND COOKING • Banana • Einkorn wheat (Triticum monococcum), now rarely grown. • Breadfruit • pasta -

Read Book in the Sweet Kitchen: the Definitive Bakers Companion Pdf
IN THE SWEET KITCHEN: THE DEFINITIVE BAKERS COMPANION PDF, EPUB, EBOOK Regan Daley | 692 pages | 01 Jul 2010 | ARTISAN | 9781579654276 | English | New York, United States In the Sweet Kitchen: The Definitive Bakers Companion PDF Book May 13, Rhi rated it it was amazing Shelves: eat-drink. Additional Product Features Dewey Edition. Just a moment while we sign you in to your Goodreads account. We have ratings, but no written reviews for this, yet. Amazon Kindle 0 editions. The recipes are delightful though. When Women Pray Hardcover T. Amazon Second Chance Pass it on, trade it in, give it a second life. Can I count this as a vegetable serving? You may also like. Return to Book Page. What makes a book so special and deserving that it gets chosen cookbook of the year? Friend Reviews. The recipes are great, with easy-to-follow instructions. List of vegetable oils. Move beyond carrots in baking to experience the larger world of sweet vegetables. The very last recipe in the book is a standard pie crust? I got tons of requests for the sugar cookie recipe after I gave them out as holiday presents. Error rating book. Bob rated it it was amazing Nov 20, Readers also enjoyed. These desserts are magic. A friend ordered a copy of this for herself and announced "Now I will have the secret baking powers you have too! Shelves: cookbooks. What makes a book so special and deserving that it gets chosen cookbook of the year? Other Editions 2. She currently lives in Toronto with her husband, three boys, and a food-phobic dog.