4.3 Distinguishing Among Atoms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chlorine Stable Isotopes in Sedimentary Systems: Does Size Matter?
Chlorine stable isotopes in sedimentary systems: does size matter? Max Coleman Jet Propulsion Laboratory, Caltech, Pasadena, California, USA ABSTRACT: Stable isotope abundances vary because of size (atomic mass) differences. The chlorine stable isotope system was one of the first described theoretically, but had a slow, disappointment-strewn develop- ment, relative to other elements. Method improvement gave only small, but significant, differences (-1 %.) in compositions of geological materials. Eventually, brines and groundwater chlorides gave larger differences (-5%0). Physical processes like diffusion and adsorption, probably are the main controls of groundwater com- positions. In contrast, the processes producing brine compositions are still enigmatic and need further work for full understanding. Recent work on anthropogenic groundwater contaminants (chlorinated aliphatic hy- drocarbons and perchlorate) shows variations resulting from manufacturing processes; implying possibilities of tracing sources. However, they are subject to microbial degradation, producing much larger fractionations (115%0),but therefore offering better possibility of monitoring natural attenuation. So atomic size is important to give isotopic fractionation and the size of fractionation matters, allowing observation of otherwise unde- tectable processes. lecular and atomic weights by weighing the gases and relating them to the weight of hydrogen. All 1 INTRODUCTION atomic weights were integer multiples the weight of This paper aims to give a short and selective -

Unit 1Moles and Equations
Cambridge University Press 978-1-107-61665-3 – Cambridge International AS and A Level Chemistry Judith Potter Peter Cann Excerpt More information Moles and equations Unit 1 Learning outcomes You should be able to: defi ne and use the terms empirical and molecular formulae and use experimental data defi ne and use relative masses based on the to fi nd them, 12C scale write and construct equations and use them in defi ne and use the mole and Avogadro’s performing mole calculations constant perform mole calculations involving solutions analyse simple mass spectra and use the data in and gases, relative atomic mass calculations This unit gathers together the basic chemistry relative isotopic mass = calculations. You may need to refer to it as you revise mass of one atom of the isotope other topics in later units. (1/12) of the mass of one atom of 12C Relative masses are a ratio of two masses and have 1.01 Relative masses no units. An atom is the smallest part of an element that can exist and still retain the identity of the element. A 1.02 The mole and the molecule is a group of atoms bonded together and the smallest portion of an element or compound that can Avogadro constant exist alone. A simple ion is an atom which has lost or To give an easy measurement of mass, a number of gained one or more electrons. Atoms, molecules and atoms is chosen so that their combined mass is equal to ions are so small that their masses are far too small the relative atomic mass in grams. -

Chromium Isotopes Detection in Their Ores with Minimal Errors
Open Access Annals of Advances in Chemistry Research Article Chromium Isotopes Detection in their Ores with Minimal Errors ISSN 2576-3768 Loai Aljerf1* and Nuha AlMasri2 1Department of Basic Sciences, Faculty of Dental Medicine, Damascus University, Mazzeh Highway, Almazzeh, Damascus, Syria 2Department of Chemistry, Faculty of Medicine, Syrian Private University, Damascus, Syria *Address for Correspondence: Loai Aljerf, De- Abstract partment of Basic Sciences, Faculty of Dental Medicine, Damascus University, Mazzeh High- The industrial production and use of chromium have grown considerably during the past way, Almazzeh, Damascus, Syria, Tel: +963-93 fi ve decades. Abundances of the chromium isotopes in terrestrial samples are identical to 34 46 993; Email: [email protected]; 0.01%. Among the dominant species of chromium, the trivalent form widely occurs in nature in [email protected] chromite ores which is extremely immobilized especially in water bodies. Samples were mixtures Submitted: 21 July 2018 of separated chromium isotopes and the calibration was made with the same species as those Approved: 03 September 2018 used in the measurements. The method had simplifi ed the conversion of the ores to chromyl Published: 04 September 2018 fl uoride since the element could be readily separated as lead chromate from the leaching of Copyright: 2018 Aljerf L, et al. This is an open chromite-sodium peroxide fusions. Isotope assay of chromyl fl uoride under certain conditions access article distributed under the Creative was measured and the measurements of chromium isotopic anomalies ratios and isotope Commons Attribution License, which permits abundance of the chromite ores have been assessed. These provided suffi cient quantitative mass unrestricted use, distribution, and reproduction spectrometric data, which were analyzed to calculate the abundance and the mean atomic mass in any medium, provided the original work is of the questioned isotopes. -

12 Natural Isotopes of Elements Other Than H, C, O
12 NATURAL ISOTOPES OF ELEMENTS OTHER THAN H, C, O In this chapter we are dealing with the less common applications of natural isotopes. Our discussions will be restricted to their origin and isotopic abundances and the main characteristics. Only brief indications are given about possible applications. More details are presented in the other volumes of this series. A few isotopes are mentioned only briefly, as they are of little relevance to water studies. Based on their half-life, the isotopes concerned can be subdivided: 1) stable isotopes of some elements (He, Li, B, N, S, Cl), of which the abundance variations point to certain geochemical and hydrogeological processes, and which can be applied as tracers in the hydrological systems, 2) radioactive isotopes with half-lives exceeding the age of the universe (232Th, 235U, 238U), 3) radioactive isotopes with shorter half-lives, mainly daughter nuclides of the previous catagory of isotopes, 4) radioactive isotopes with shorter half-lives that are of cosmogenic origin, i.e. that are being produced in the atmosphere by interactions of cosmic radiation particles with atmospheric molecules (7Be, 10Be, 26Al, 32Si, 36Cl, 36Ar, 39Ar, 81Kr, 85Kr, 129I) (Lal and Peters, 1967). The isotopes can also be distinguished by their chemical characteristics: 1) the isotopes of noble gases (He, Ar, Kr) play an important role, because of their solubility in water and because of their chemically inert and thus conservative character. Table 12.1 gives the solubility values in water (data from Benson and Krause, 1976); the table also contains the atmospheric concentrations (Andrews, 1992: error in his Eq.4, where Ti/(T1) should read (Ti/T)1); 2) another category consists of the isotopes of elements that are only slightly soluble and have very low concentrations in water under moderate conditions (Be, Al). -

Determination of Picogram Quantities of Vanadium in Calcite And
+ + Anal. Chem. 1996, 68, 371-377 Determination of Picogram Quantities of Vanadium in Calcite and Seawater by Isotope Dilution Inductively Coupled Plasma Mass Spectrometry with Electrothermal Vaporization David W. Hastings* and Steven R. Emerson School of Oceanography, WB-10, University of Washington, Seattle, Washington 98195 Bruce K. Nelson Department of Geological Sciences, AJ-20, University of Washington, Seattle, Washington 98195 We developed a method to measure picogram quantities Recent interest in the geochemistry of vanadium has arisen of vanadium in calcite and seawater by isotope dilution from its unique redox behavior 9,10 and the observation of enriched (ID) inductively coupled plasma mass spectrometry using concentrations in anoxic sediments, black shale, and crude oil.11,12 electrothermal vaporization (ETV) to introduce the sample Because V deposition is redox sensitive, its concentration in into the plasma. A 50V isotope spike enriched to 44 atom seawater is most likely sensitive to the oxidation state of sedi- % was equilibrated with samples, followed by chemical ments. The calcitic shells of foraminifera accurately record purification by cation exchange chromatography. Samples seawater concentrations of V,13 so the V content of the fossil shells were introduced into the ETV unit with a Pd modifier and of these microscopic oceanic organisms potentially can serve as heated to 1000 °C. This quantitatively eliminates the an indicator of changes in past ocean anoxia.14 ClO+ isobaric interference with V at m/z 51 for solutions A typical 5 mg hand-picked sample of these microscopic shells up to 0.5 N HCl. The procedural blank was 0.27 pg of yields only 50-200 pg of vanadium. -
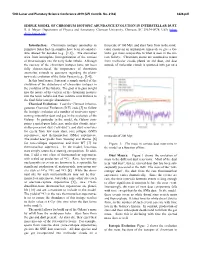
Simple Model of Chromium Isotopic Abundance Evolution in Interstellar Dust
50th Lunar and Planetary Science Conference 2019 (LPI Contrib. No. 2132) 3226.pdf SIMPLE MODEL OF CHROMIUM ISOTOPIC ABUNDANCE EVOLUTION IN INTERSTELLAR DUST. B. S. Meyer,. Department of Physics and Astronomy, Clemson University, Clemson, SC 29634-0978, USA (mbra- [email protected]). Introduction: Chromium isotopic anomalies in timescale of 100 Myr, and stars form from in the mole- primitive Solar System samples have been of consider- cular clouds on an appropriate timescale to give a Ga- able interest for decades (e.g., [1,2]). The anomalies lactic gas mass comparable to what is seen in the cur- arise from incomplete homogenization of the carriers rent Galaxy. Chromium atoms are assumed to return of these isotopes into the early Solar nebula. Although from molecular clouds plated on old dust, and dust the carriers of the chromium isotopes have not been outside of molecular clouds is sputtered into gas on a fully characterized, the importance of chromium anomalies extends to questions regarding the plane- tary-scale evolution of the Solar System (e.g., [3,4]). In this brief paper, I present a simple model of the evolution of the abundances of chromium isotopes in the evolution of the Galaxy. The goal is to gain insight into the nature of the carriers of the chromium isotopes into the Solar nebula and their relative contributions to the final Solar isotopic abundances. Chemical Evolution: I use the Clemson Inhomo- geneous Chemical Evolution (ICE) code [5] to follow the isotopic evolution of a number of reservoirs repre- senting interstellar dust and gas in the evolution of the Galaxy. -

Chromium Isotope Behaviour in Natural Waters
UNIVERSITY OF SOUTHAMPTON FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES Ocean and Earth Sciences Chromium Isotope Behaviour in Natural Waters by Heather Jane Goring-Harford Thesis for the degree of Doctor of Philosophy June 2017 UNIVERSITY OF SOUTHAMPTON ABSTRACT FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES Ocean and Earth Sciences Doctor of Philosophy CHROMIUM ISOTOPE BEHAVIOUR IN NATURAL WATERS By Heather Jane Goring-Harford The isotopes of chromium (Cr) fractionate during terrestrial oxidation reactions that require the presence of oxygen. The main source of Cr to the oceans is via rivers, and thus Cr isotopic signatures (expressed as δ53Cr) preserved in authigenic sediments are increasingly being used to reconstruct the oxygen levels of ancient environments. However, Cr can undergo various reactions in natural waters that may fractionate Cr isotopes. The contribution of these reactions to authigenic sediment δ53Cr values is not well understood, and this limits the interpretation of δ53Cr values measured in ancient archives. This thesis describes the development of a method to accurately and precisely measure Cr isotopic signatures in natural waters with extremely low Cr concentrations, and evaluates the behaviour of Cr isotopes in water samples from a range of environmental settings. Seawater samples from the Atlantic Ocean Oxygen Minimum Zone (OMZ) were analysed to evaluate whether Cr isotopic fractionation was enhanced under depleted oxygen conditions. Results indicate that the Atlantic OMZ is not sufficiently depleted in oxygen to reduce and remove Cr from seawater, although δ53Cr values (1.08 – 1.72‰) were variable due to adsorption of Cr(III) to particles on the shelf, and hydrological mixing. Black Sea seawater samples that had dissolved oxygen concentrations close to zero were enriched in 52Cr, resulting in a δ53Cr that was 0.38‰ lower than the overlying fully oxygenated waters. -

Accelerator Mass Spectrometry of 36Cl and 129I Analytical Aspects and Applications
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1 Accelerator Mass Spectrometry of 36Cl and 129I Analytical Aspects and Applications BY VASILY ALFIMOV ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 UPPSALA ISBN 91-554-6124-7 2005 urn:nbn:se:uu:diva-4725 Dissertation presented at Uppsala University to be publicly examined in Häggsalen, The Ång- ström Laboratory, Uppsala, Friday, January 21, 2005 at 10:15 for the Degree of Doctor of Philosophy. The examination will be conducted in English. Abstract Alfimov, V. 2005. Accelerator Mass Spectrometry of 36Cl and 129I: Analytical Aspects and Applications. Acta Universitatis Upsaliensis. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1. 81 pp. Uppsala. ISBN 91-554-6124-7 36 129 Two long-lived halogen radionuclides ( Cl, T1/2 = 301 kyr, and I, T1/2 = 15.7 Myr) have been studied by means of Accelerator Mass Spectrometry (AMS) at the Uppsala Tandem Labo- ratory. The 36Cl measurements in natural samples using a medium-sized tandem accelerator (∼1 MeV/amu) have been considered. A gas-filled magnetic spectrometer (GFM) was proposed for the separation of 36Cl from its isobar, 36S. Semi-empirical Monte-Carlo ion optical calculations were conducted to define optimal conditions for separating 36Cl and 36S. A 180° GFM was constructed and installed at the dedicated AMS beam line. 129I has been measured in waters from the Arctic and North Atlantic Oceans. Most of the 129I currently present in the Earth’s surface environment can be traced back to liquid and gaseous releases from the nuclear reprocessing facilities at Sellafield (UK) and La Hague (France). -

Technical and Regulatory Guidance Environmental Molecular Diagnostics
Technical and Regulatory Guidance Environmental Molecular Diagnostics New Site Characterization and Remediation Enhancement Tools April 2013 Prepared by The Interstate Technology & Regulatory Council Environmental Molecular Diagnostics Team ABOUT ITRC The Interstate Technology and Regulatory Council (ITRC) is a public-private coalition working to reduce bar- riers to the use of innovative environmental technologies and approaches so that compliance costs are reduced and cleanup efficacy is maximized. ITRC produces documents and training that broaden and deepen technical knowledge and expedite quality regulatory decision making while protecting human health and the envir- onment. With private and public sector members from all 50 states and the District of Columbia, ITRC truly provides a national perspective. More information on ITRC is available at www.itrcweb.org. ITRC is a pro- gram of the Environmental Research Institute of the States (ERIS), a 501(c)(3) organization incorporated in the District of Columbia and managed by the Environmental Council of the States (ECOS). ECOS is the national, nonprofit, nonpartisan association representing the state and territorial environmental commissioners. Its mission is to serve as a champion for states; to provide a clearinghouse of information for state envir- onmental commissioners; to promote coordination in environmental management; and to articulate state pos- itions on environmental issues to Congress, federal agencies, and the public. DISCLAIMER This material was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any war- ranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or use- fulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. -

ILPAC Unit S2: Atomic Structure
UNIT INDEPENDENT LEARNING PROJECT FOR ADVANCED CHEMISTRY Periodic Table of the Elements o 2 I He II ill] III IV V VI VIII 4.0 3 4 5 6 7 8 9 10 Li Be B C N 0 F Ne 6.9 9.0 10.8 12.0 14.0 16.0 19.0 20.2 11 12 13 14 15 16 17 18 Na Mg Al Si P S CI Ar 23.0 24.3 27.0 28.1 31.0 32.1 35.5 39.9 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 3! 36 K Ca Sc Ti V Cr Mn Fe Co Ni eu Zn Ga Ge As Se BrlKr 39.1 40.1 45.0 47.9 50.9 52.0 54.9 55.9 58.9 58.7 63.5 65.4 69.7 72.6 74.9 79.0 79 83.8 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 S4 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 85.5 87.6 88.9 91.2 92.9 95.9 99.0 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.91 ' 3 ' . 3 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba La 4 Hf Ta W Re Os If Pt Au Hg Tl Pb Bi Po AtlRn 132.9 137.3 138.9 178.5 181.0 183.9 186.2 190.2 192.2 195.1 197.0 200.6 204.4 207.2 209.0 210.0 210.01222.0 87 88 89 Fr Ra Ac~ 223.0 226.0 227.0 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 140.1 140.9 144.2 (147) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 175.0 90 91 92 93 94 95 96 97 98 99 100 101 102 103 "--- Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lw 232.0 231.0 238.1 (237) 239.1 (243) (241) (247) (251 ) (254) (253) (256) ( 254) (257) A value in brackets denotes the mass number of the most stable isotope. -

The Isotope Effect: Prediction, Discussion, and Discovery
1 The isotope effect: Prediction, discussion, and discovery Helge Kragh Centre for Science Studies, Department of Physics and Astronomy, Aarhus University, 8000 Aarhus, Denmark. ABSTRACT The precise position of a spectral line emitted by an atomic system depends on the mass of the atomic nucleus and is therefore different for isotopes belonging to the same element. The possible presence of an isotope effect followed from Bohr’s atomic theory of 1913, but it took several years before it was confirmed experimentally. Its early history involves the childhood not only of the quantum atom, but also of the concept of isotopy. Bohr’s prediction of the isotope effect was apparently at odds with early attempts to distinguish between isotopes by means of their optical spectra. However, in 1920 the effect was discovered in HCl molecules, which gave rise to a fruitful development in molecular spectroscopy. The first detection of an atomic isotope effect was no less important, as it was by this means that the heavy hydrogen isotope deuterium was discovered in 1932. The early development of isotope spectroscopy illustrates the complex relationship between theory and experiment, and is also instructive with regard to the concepts of prediction and discovery. Keywords: isotopes; spectroscopy; Bohr model; atomic theory; deuterium. 1. Introduction The wavelength of a spectral line arising from an excited atom or molecule depends slightly on the isotopic composition, hence on the mass, of the atomic system. The phenomenon is often called the “isotope effect,” although E-mail: [email protected]. 2 the name is also used in other meanings. -

45024017.Pdf
Ain Shams University Faculty of Science Radiochemical Study on the Separation of Chromium-51 from the Irradiated Target by Using Commercial and/or Synthesized Ion Exchanger A thesis Submitted by Mohamed Ismail Mohamed Mohamed Aydia (M. Sc. Inorganic and Physical Chemistry 2007) Chemist\ Radioisotopes Production Facility Egyptian Second Research Reactor Atomic Energy Authority For the Degree of Doctor of Philosophy in Science (Chemistry). Department of Chemistry Faculty of Science Ain Shams University (2012) Ain Shams University Faculty of Science Approval Sheet The Thesis entitled Radiochemical Study on the Separation of Chromium-51 from the Irradiated Target by Using Commercial and/or Synthesized Ion Exchanger Submitted by/ Mohamed Ismail Mohamed Aydia (M. Sc. Inorganic and Physical Chemistry 2007) For the Degree of Doctor of Philosophy in Science (Chemistry). This thesis has been approved by supervisor committee SUPERVISORS • Prof. Dr. M.S. Abdel-Mottaleb ….……………… Professor of Inorganic Chemistry Faculty of Science-Ain Shams University • Prof. Dr. Salah Soliman ….……………… Ex-Chairman of Hot Labs Center Supervisor of Radioisotopes Production Facility Atomic Energy Authority • Prof. Dr. A. A. El-Mohty ….……………… Professor of Radiochemistry Radioactive Isotopes and Generator Dep. Atomic Energy Authority • Prof. K.M. El-Azony ….……………… Associate Professor of Radiochemistry Radioactive Isotopes and Generator Dep. Atomic Energy Authority. Prof. Dr. Maged Shafik Antonious Head of Chemistry Department Faculty of Science - Ain Shams University CHAPTER (I) INTRODUCTION CHAPTER (II) EXPERIMENTAL CHAPTER (III) RESULTS AND DISCUSSION REFERENCES ] SUMMARY ARABIC SUMMARY ACKNOWLEDGEMENT I would like to express my gratitude to Prof. Dr. Mohamed Sabry Abdel-Mottaleb, Professor of Inorganic Chemistry, Faculty of Science, Ain Shams University for his supervision and his sponsorship for this work.