En Este Número
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COVID-19 Vaccination Programme: Information for Healthcare Practitioners
COVID-19 vaccination programme Information for healthcare practitioners Republished 6 August 2021 Version 3.10 1 COVID-19 vaccination programme: Information for healthcare practitioners Document information This document was originally published provisionally, ahead of authorisation of any COVID-19 vaccine in the UK, to provide information to those involved in the COVID-19 national vaccination programme before it began in December 2020. Following authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency being given to the COVID-19 Vaccine Pfizer BioNTech on 2 December 2020, the COVID-19 Vaccine AstraZeneca on 30 December 2020 and the COVID-19 Vaccine Moderna on 8 January 2021, this document has been updated to provide specific information about the storage and preparation of these vaccines. Information about any other COVID-19 vaccines which are given regulatory approval will be added when this occurs. The information in this document was correct at time of publication. As COVID-19 is an evolving disease, much is still being learned about both the disease and the vaccines which have been developed to prevent it. For this reason, some information may change. Updates will be made to this document as new information becomes available. Please use the online version to ensure you are accessing the latest version. 2 COVID-19 vaccination programme: Information for healthcare practitioners Document revision information Version Details Date number 1.0 Document created 27 November 2020 2.0 Vaccine specific information about the COVID-19 mRNA 4 Vaccine BNT162b2 (Pfizer BioNTech) added December 2020 2.1 1. -
Storage Best Practices for Frozen Vaccines-Fahrenheit
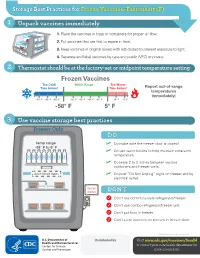
Storage Best Practices for Frozen Vaccines–Fahrenheit (F) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -70° F -65° F -60° F -50° F -45° F -40° F -35° F 10° F 15° F -58° F 5° F 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -58° F to 5° F ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision December 2020 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. Stand-alone D. -

CDC Vaccine Storage and Handling Guide
Table of Contents General Information Vaccine Storage and Handling Best Practices 5 Selected Biologicals Diphtheria Toxoid-, Tetanus Toxoid- and acellular Pertussis-Containing Vaccines DTaP: DAPTACEL, Infanrix, Tripedia 11 DTaP-IPV: KINRIX 11 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV/Hib: Pentacel 11 Haemophilus influenzae type b-Containing Vaccines Hib: ActHIB, Hiberix, PedvaxHIB 15 Hib-HepB: Comvax 15 DTaP-IPV/Hib: Pentacel 11 Hepatitis-Containing Vaccines HepA: Havrix, VAQTA 19 HepB: Engerix-B, Recombivax HB 19 HepA-HepB: Twinrix 19 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-HepB-IPV: Pediarix 11 Hib-HepB: Comvax 15 Human Papillomavirus Vaccines HPV2: Cervarix 23 HPV4: Gardasil 23 Vaccine Storage and Handling Guide —————————————————————————————— Page 2 Table of Contents Influenza Vaccines LAIV: FluMist 27 TIV: Afluria, Fluarix, FluLaval, Fluvirin, Fluzone, Fluzone High-Dose, Fluzone Intradermal 29 Measles-, Mumps- and Rubella-Containing Vaccine MMR: M-M-RII 33 MMRV: ProQuad 69 Meningococcal Vaccines MCV4: Menactra, Menveo 37 MPSV4: Menomune 41 Pneumococcal Vaccines PCV13: Prevnar 13 45 PPSV23: Pneumovax 23 45 Poliovirus-Containing Vaccine IPV: IPOL 49 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV: KINRIX 11 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-IPV/Hib: Pentacel 11 Rotavirus Vaccines RV1: ROTARIX 53 RV5: RotaTeq 53 Tetanus Toxoid Vaccine TT: Tetanus Toxoid 57 Vaccine Storage and Handling Guide —————————————————————————————— -

The Falling Netanyahu Takes Down a Cantankerous Political Class
WWW.TEHRANTIMES.COM I N T E R N A T I O N A L D A I L Y 8 Pages Price 50,000 Rials 1.00 EURO 4.00 AED 43rd year No.13954 Saturday MAY 29, 2021 Khordad 8, 1400 Shawwal 17, 1442 Elections is Brighton’s Alireza Steel ingot export Germany recognizes competition to Jahanbakhsh to decide rises 135% in colonial-era massacres in serve people Page 2 on his future Page 3 a month on year Page 4 Namibia as genocide Page 5 Unity is the secret behind the Resistance’s victories: Amir-Abdollahian TEHRAN - Hossein Amir-Abdollahian, the resistance and steadfastness victory could special aide to the speaker of the Iranian be achieved. This victory sent an important Parliament on international affairs, has re- message that the Zionist enemy only under- flected on the secret behind the recent victory stands the language of force and resistance,” of the Palestinian resistance against Israel. he told the Lebanese Al-Ahed News. Amir-Abdollahian said the 2006 victo- The Iranian diplomat added, “This ry of the Lebanese resistance movement victory and other victories achieved by against Israel raised faith in the resistance the resistance, especially the victory of and sent a message that Israel understands July 2006, were achieved in light of the only the language of power. unity of the Lebanese people and the gold- “The flight of the Zionist entity from en equation in Lebanon – the army, the southern Lebanon raised faith in the resist- people, and the resistance. Crabs in ance among the Lebanese, and that through Continued on page 3 Tehran, Moscow confer on joint investment in agricultural sector TEHRAN – Iranian Agriculture Minis- investment in various agricultural fields. -

Outdoor, Online Activities Mark National Sport
BUSINESS | Page 1 SPORT | Page 1 Envoy affi rms US Bayern Munich fi rms’ support for eye sixth title aft er 2030 Qatar Vision, reaching Club jobs creation World Cup fi nal published in QATAR since 1978 WEDNESDAY Vol. XXXXI No. 11820 February 10, 2021 Jumada II 28, 1442 AH GULF TIMES www. gulf-times.com 2 Riyals His Highness the Amir Sheikh Tamim bin Hamad al-Thani celebrated Qatar’s National Sport Day at Al Bidda Park yesterday. His Highness the Amir also wished everyone an enjoyable day through a tweet, whose unoff icial translation is as follows: “On our Sport Day during this exceptional year that all countries of the world are experiencing, exercise remains an indispensable healthy and social behaviour; wishing everyone an enjoyable day.” Doha Plan of Action Defence minister opens world’s to uplift poor nations Outdoor, online activities largest calisthenics park The Doha Plan of Action will be a starting point for transforming the lives of millions of people living in the most vulnerable countries, mark National Sport Day and putting the least developed countries on the path towards sustainable and comprehensive t was a National Sport Day (NSD) stances, in addition to being a highly development, HE the Permanent 2021 with a diff erence as people eff ective activity in terms of fi tness Representative of Qatar to the Iaround Qatar celebrated the occa- and well-being. United Nations Ambassador Sheikha sion yesterday by observing Covid-19 Others were seen running, jog- Alya Ahmed bin Saif al-Thani, has precautionary and preventive meas- ging, roller skating or simply walking, said. -

Asia COVID-19 & Vaccine Tracker
9 July 2021 Free to View Asia COVID-19 & Vaccine Tracker Economics - Asia Tougher restrictions As COVID-19 cases continue to surge across Asia, more Yun Liu authorities have re-imposed restrictions or even lockdowns Economist The Hongkong and Shanghai Banking Corporation Limited But this has increased the urgency to ramp up vaccine Frederic Neumann procurement and roll-out in the region Co-Head of Asian Economics Research The Hongkong and Shanghai Banking Corporation Limited Some economies have started pilot programmes to revive tourism, but a full re-opening may take a long time Maitreyi Das Associate Bangalore Déjà vu No end in sight just yet. The Delta variant is causing daily new COVID-19 cases to surge to new record highs across Asia, prompting more governments to re-impose tougher restrictions. This is particularly evident in ASEAN, where cases in Indonesia and Vietnam have been rising sharply. The former has extended its tightening measures throughout the country until 20 July, while the latter will impose a strict lockdown in Ho Chi Minh City (HCMC) from 9 July for 15 days. Meanwhile, just two weeks before the Tokyo Olympics, Japan is set to declare its fourth state of emergency in Tokyo from 12 July to 20 August, raising the possibility of no spectators attending the games. Meanwhile, Singapore is the only country that has decided to ease its social restrictions from 12 July, as new cases remain low. Urgency on vaccination The recent outbreaks in the region has alerted the authorities that the key to exiting the pandemic is to accelerate vaccine procurement and roll-out. -

COVID-19 Vaccine Storage & Handling
COVID-19 Vaccine Storage & Handling Brooke Zeringue, Adrienne Whitney, & Robert Starszak Louisiana Department of Health Office of Public Health Immunization Program • Everyone (16 years and older) can be vaccinated. Louisiana Order COVID-19 vaccine in LINKS. Second COVID-19 doses are not automatically ordered. You must Vaccination order every dose you need. Guidelines All vaccine doses administered must be entered into LINKS within 24 hours and inputted as administered, NOT historical. COVID-19 Vaccine Delivery Storage & Handling for Moderna COVID-19 Vaccine: Direct from McKesson Vaccine will arrive frozen Larger quantities Thermal Shipper Storage & Handling for Pfizer- BioNTech COVID-19 Vaccine: Direct from Shipped in thermal shipping container Pfizer Vaccine arrives frozen Larger quantities Storage & Handling for Moderna COVID-19 Vaccine: Morris & Dickson Vaccine will arrive thawed Smaller Quantities Johnson & Johnson (Janssen) COVID-19 Vaccine Vaccine Type: • Viral Vector Vaccine Age indication: Overview of • 18 years or older. the Johnson & Dose & Route: Johnson • 0.5 mL; intramuscular COVID-19 Schedule: Vaccine • Single dose Dose Preparation: • 5 doses per vial Johnson & Johnson | STORING VACCINE VIALS Refrigerator 2°C to 8°C (36°F to 46°F) • Store up to the expiration date • DO NOT store frozen • Protect from light • You can find the expiration date at vaxcheck.jnj Johnson & Johnson | ADMINISTERING • Keep refrigerated until thawed (if arrived frozen). Refrigerator • PUNCTURED VIALS: Viable up to 6 hours 2°C to 8°C (36°F to 46°F) • If arrived frozen and needed immediately, thaw for Room 1 to 2 hours. • UNPUNCTURED VIALS: Viable up to 12 hours kept Temperature at 9°C to 25°C (47°F to 77°F) • PUNCTURED VIALS: Viable up to 2 hours Up to 25°C (Up to 77°F) Johnson & Johnson | ADMINISTERING Visually inspect the Before withdrawing each Johnson & Johnson COVID- dose of vaccine, carefully 19 vaccine vials for other mix the contents by Each dose must contain 0.5 particulate matter and/or swirling gently in an upright mL of vaccine. -

Bibi's Big Mistake: Fall of Fake Regime?
WWW.TEHRANTIMES.COM I N T E R N A T I O N A L D A I L Y 8 Pages Price 50,000 Rials 1.00 EURO 4.00 AED 43rd year No.13941 Wednesday MAY 12, 2021 Ordibehesht 22, 1400 Ramadan 29, 1442 Iran: Tehran-Riyadh Daei, Hejazi the best Blood donation dialogue conducted by Iranian players of up 27% during Felicitation special envoys Page 2 century: IFFHS Page 3 Qadr nights Page 7 on Eid-al Fitr Iran rejects Pentagon’s claim, denounces U.S. ‘unprofessional’ behavior in Hormuz Bibi’s big mistake: Fall TEHRAN - The Islamic Revolutionary committing “provocative, gratuitous and Guards Corps Navy has reacted to a claim unprofessional behaviors such as flying heli- by the Pentagon that the IRGC speed- copters, firing flares and aimless shooting.” boats unprofessionally came close to an The statement said the IRGC boats See page 3 American vessel. maintained a legal distance from the The IRGC Navy said in a statement on American vessels in accordance with of fake regime? Tuesday that IRGC boats did not act unpro- international maritime regulations and fessionally and while they were conducting warned them against “dangerous and a regular and conventional operation, they unprofessional behavior.” encountered seven American Navy vessels Continued on page 3 Iranian COVID-19 Electricity projects worth over $320m vaccine enters large- put into operation TEHRAN – Iranian Energy Minister projects, as well as installing new PV sys- Reza Ardakanian inaugurated major tems for nomadic households. scale production phase electricity projects worth 13.45 trillion The national electricity network’s rials (about $320.2 million) across the new dispatching center which has been country on Tuesday, in the sixth week of completed with 11.44 trillion rials (about the ministry’s A-B-Iran program in the $272.3 million) of investment is using current Iranian calendar year (started on world’s latest technologies in Energy March 21). -

En Este Número
Boletín Científico No. 18 (1-10 julio/2021) EN ESTE NÚMERO VacCiencia es una publicación dirigida a Resumen de candidatos vacu- investigadores y especialistas dedicados a nales contra la COVID-19 ba- la vacunología y temas afines, con el ob- sadas en la plataforma de sub- jetivo de serle útil. Usted puede realizar unidad proteica en desarrollo a sugerencias sobre los contenidos y de es- nivel mundial. (segunda parte) ta forma crear una retroalimentación Artículos científicos más que nos permita acercarnos más a sus recientes de Medline sobre necesidades de información. vacunas. Patentes más recientes en Patentscope sobre vacunas. Patentes más recientes en USPTO sobre vacunas. 1| Copyright © 2020. Todos los derechos reservados | INSTITUTO FINLAY DE VACUNAS Resumen de vacunas contra la COVID-19 basadas en la plataforma de subunidad proteica en desarrollo a nivel mundial (segunda parte) Las vacunas de subunidades antigénicas son aquellas en las que solamente se utilizan los fragmentos específicos (llamados «subunidades antigénicas») del virus o la bacteria que es indispensable que el sistema inmunitario reconozca. Las subunidades antigénicas suelen ser proteínas o hidratos de carbono. La mayoría de las vacunas que figuran en los calendarios de vacunación infantil son de este tipo y protegen a las personas de enfermedades como la tos ferina, el tétanos, la difteria y la meningitis meningocócica. Este tipo de vacunas solo incluye las partes del microorganismo que mejor estimulan al sistema inmunitario. En el caso de las desarrolladas contra la COVID-19 contienen generalmente, la proteína S o fragmentos de la misma como el Dominio de Unión al Receptor (RBD, por sus siglas en inglés). -

INTERNATIONAL STUDENT COVID VACCINE LIST and COMMUNICATION Dear International Student
INTERNATIONAL STUDENT COVID VACCINE LIST and COMMUNICATION Dear International Student We have provided a list of Selected Countries to help you find the COVID-19 vaccine you may have already received. Please see the “WHO” list below to find your COVID-19 vaccine, and if it has been APPROVED. And please upload your record following these directions: https://youtu.be/wvBGeRqIMHc If you see that your vaccine HAS NOT yet been approved by “WHO”, you may be required to receive an approved COVID-19 vaccine and upload those records to meet UC campus policy and requirements. Many US Airports are now providing COVID-19 vaccine on-site. Here is a list of several California Airports that you may arriving where you may receive a vaccine IMMEDIATELY upon your entry into the U.S. LAX – Los Angeles https://www.flylax.com/TravelSafely SNA – Orange County https://hoagurgentcare.com/airport/ SFO – San Francisco https://www.flysfo.com/travel-well/vaccination-site-sfo If you have any additional questions or concerns, please call (949)824-2300 for support. THANK YOU for helping to ensure UCI continues to be a SAFE and HEALTHY Campus! Dr Chang INDIA SOUTH KOREA TAIWAN INDONESIA VIETNAM JAPAN Vaccine Vaccine Vaccine Vaccine Vaccine Vaccine Oxford–AstraZeneca Oxford-AstraZeneca Sinovac Sputnik V Pfizer–BioNTech Covishield Pfizer–BioNTech Oxford–AstraZeneca Oxford–AstraZeneca Sinopharm Janssen Sinopharm Moderna Pfizer–BioNTech Moderna Pfizer–BioNTech Moderna Moderna Covaxin Novavax Moderna Johnson & Johnson Oxford–AstraZeneca Sputnik V Novavax Nanocovax Johnson & Johnson Sputnik V COVIVAC Sputnik V CanSino Vabiotech MVC-COV1901 ZyCoV-D Corbevax Covovax . -

31St May-6Th June Weekly Compilation
31st May -6th June Weekly Compilation (The Hindu+ Indian Express + PIB + Other World Wide News) PM CARES FOR CHILDREN SCHEME (Source: The HINDU) Why in News: The Prime Minister has announced a special PM-CARES for Children scheme. The scheme includes a comprehensive financial aid package for children orphaned during the pandemic. PM-CARES for Children Scheme The PM-CARES for Children Scheme will support children who have lost both parents or surviving parent or legal guardian/adoptive parents due to Covid-19. Features of the PM-CARES for Children Scheme Fixed Deposit in the name of the child . PM CARES will create a corpus of Rs 10 lakh for each child when s/he reaches 18 years of age. This corpus will be used to give monthly financial support from 18 years of age for the next five years. On reaching the age of 23 years, he or she will get the corpus amount as one lump sum for personal and professional use. School Education: For children under 10 years . The child will be given admission to the nearest Kendriya Vidyalaya or in a private school as a day scholar. If the child is admitted to a private school, the fees as per the Right to Education(RTE) norms will be given from the PM CARES. School Education: for children between 11-18 years: . The child will be given admission to any Central Government residential school such as Sainik School, Navodaya Vidyalaya etc. In case the child is to be continued under the care of Guardian. Then s/he will be given admission to the nearest Kendriya Vidyalaya or in a private school as a day scholar. -

Coronavirus: Quello Che C'è Da Sapere – 7 Giugno 2021
Coronavirus: quello che c’è da sapere – 7 giugno 2021 Sommario Quando è iniziata l’epidemia? ...................................................................2 Come viene diagnosticata la malattia Covid-19? ...................................23 Quando è arrivata in Italia? .......................................................................2 Che cosa sono i test sierologici? a cosa servono? ................................25 A cosa è dovuta l’infezione? .....................................................................2 Chi viene colpito dalla malattia Covid-19? ............................................25 Quanto è diffusa l’epidemia? ....................................................................2 Quanto è letale il virus? Quali sono i fattori di rischio? .........................25 Che cosa sono i coronavirus? ...................................................................2 Quali sono le conseguenze a medio e lungo termine del Covid-19? .....29 Qual è l’origine del virus? .........................................................................4 Il virus può mutare? ..................................................................................4 Esiste un vaccino? ...................................................................................31 Come si trasmette l’infezione? .................................................................9 Quali sono le terapie disponibili?............................................................46 I bambini sono più soggetti all’infezione? .............................................13