22.5 Condensation Reactions Involving Ester Enolate Ions 1073
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diversity-Oriented Combinatorial Biosynthesis of Benzenediol Lactone Scaffolds by Subunit Shuffling of Fungal Polyketide Synthases
Diversity-oriented combinatorial biosynthesis of benzenediol lactone scaffolds by subunit shuffling of fungal polyketide synthases Yuquan Xua,b,1, Tong Zhouc,1, Shuwei Zhangc, Patricia Espinosa-Artilesb, Luoyi Wangc, Wei Zhanga, Min Lina, A. A. Leslie Gunatilakab,d, Jixun Zhanc,2, and István Molnárb,d,2 aBiotechnology Research Institute, The Chinese Academy of Agricultural Sciences, Beijing 100081, P. R. China; bNatural Products Center, School of Natural Resources and the Environment, University of Arizona, Tucson, AZ 85706; cDepartment of Biological Engineering, Utah State University, Logan, UT 84322; and dBio5 Institute, University of Arizona, Tucson, AZ 85721 Edited by Jerrold Meinwald, Cornell University, Ithaca, NY, and approved June 23, 2014 (received for review April 16, 2014) Combinatorial biosynthesis aspires to exploit the promiscuity of biosynthesis inhibitory activities in animals. 10,11-dehydrocurvularin microbial anabolic pathways to engineer the synthesis of new (7;Fig.1)isaDALwitha12-memberedring(DAL12)that chemical entities. Fungal benzenediol lactone (BDL) polyketides modulates the heat shock response and the immune system (8, 9). are important pharmacophores with wide-ranging bioactivities, BDL scaffolds are biosynthesized by pairs of collaborating, including heat shock response and immune system modulatory sequentially acting iterative polyketide synthases (iPKSs) (3) – effects. Their biosynthesis on a pair of sequentially acting iterative forming quasi-modular BDL synthases (BDLSs) (Fig. 1) (11 14). polyketide synthases (iPKSs) offers a test case for the modulariza- Each of the BDLS subunits catalyze recursive, decarboxylative tion of secondary metabolic pathways into “build–couple–pair” Claisen condensations of malonyl-CoA using a single core set of ketoacyl synthase (KS), acyl transferase (AT), and acyl carrier combinatorial synthetic schemes. -

Experiment 19 — Aldol Condensation
Chem 22 Spring 2010 Experiment 19 — Aldol Condensation _____________________________________________________________________________ Pre-lab preparation. (1) Write the mechanism of the base-catalyzed aldol condensation of acetone and a generalized aromatic aldehyde, Ar–CH=O to give the α,β-unsaturated product (i.e. the reaction at the top of the next page, but with a 1:1 ratio of ketone and aldehyde). Remember that dehydration in this case occurs under basic conditions, so it can't start with protonation of the hydroxyl group. Nor can it go via an E2 pathway. (2) Draw the structures of all the possible aldehyde and ketone reactants (not the 25 possible condensation products!). (3) The new CC double bonds of the condensation products are E rather than Z, as shown in the acetone example. Why? (4) What's the purpose of rinsing the crude product with dilute acetic acid, followed by ethanol? (5) What is the procedure for carrying out a single-solvent recrystallization? Write this out in detail. The aldol condensation has historically been one of the favorite tools in the synthetic organic chemist's repertoire because of its versatility in forming new CC bonds. Since its discovery in the 1870s the aldol condensation has been use extensively in the synthesis of natural products and other complex molecules. In a typical base-catalyzed aldol condensation an enolate ion attacks the carbonyl group of an aldehyde or ketone. This carbonyl addition produces a β-hydroxy carbonyl compound. In many cases the initially formed condensation product undergoes an "E1cB" dehydration to produce an α,β-unsaturated carbonyl compound as the final product. -

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

(12) Patent Application Publication (10) Pub. No.: US 2005/0044778A1 Orr (43) Pub
US 20050044778A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0044778A1 Orr (43) Pub. Date: Mar. 3, 2005 (54) FUEL COMPOSITIONS EMPLOYING Publication Classification CATALYST COMBUSTION STRUCTURE (51) Int. CI.' ........ C10L 1/28; C1OL 1/24; C1OL 1/18; (76) Inventor: William C. Orr, Denver, CO (US) C1OL 1/12; C1OL 1/26 Correspondence Address: (52) U.S. Cl. ................. 44/320; 44/435; 44/378; 44/388; HOGAN & HARTSON LLP 44/385; 44/444; 44/443 ONE TABOR CENTER, SUITE 1500 1200 SEVENTEENTH ST DENVER, CO 80202 (US) (57) ABSTRACT (21) Appl. No.: 10/722,127 Metallic vapor phase fuel compositions relating to a broad (22) Filed: Nov. 24, 2003 Spectrum of pollution reducing, improved combustion per Related U.S. Application Data formance, and enhanced Stability fuel compositions for use in jet, aviation, turbine, diesel, gasoline, and other combus (63) Continuation-in-part of application No. 08/986,891, tion applications include co-combustion agents preferably filed on Dec. 8, 1997, now Pat. No. 6,652,608. including trimethoxymethylsilane. Patent Application Publication Mar. 3, 2005 US 2005/0044778A1 FIGURE 1 CALCULATING BUNSEN BURNER LAMINAR FLAME VELOCITY (LFV) OR BURNING VELOCITY (BV) CONVENTIONAL FLAME LUMINOUS FLAME Method For Calculating Bunsen Burner Laminar Flame Velocity (LHV) or Burning Velocity Requires Inside Laminar Cone Angle (0) and The Gas Velocity (Vg). LFV = A, SIN 2 x VG US 2005/0044778A1 Mar. 3, 2005 FUEL COMPOSITIONS EMPLOYING CATALYST Chart of Elements (CAS version), and mixture, wherein said COMBUSTION STRUCTURE element or derivative compound, is combustible, and option 0001) The present invention is a CIP of my U.S. -

Aldol Condensation- Aldehyde (Or Ketone) Enolate Condenses with Aldehyde (Or Ketone)
Chem 232 Summary of Alpha Substitutions page 1 Aldol Condensation- aldehyde (or ketone) enolate condenses with aldehyde (or ketone): O CH O O H 3 H CH 3 CH3 C C C C CH C C CH2 CH2 H -H O H H O OH 2 H nucleophile electrophile -hydroxy aldehyde -unsaturated aldehyde The nucleophile can be a ketone enolate or aldehyde enolate and the electrophile (shaded) can be an aldehyde or ketone. Crossed Aldol- Condensation between two different carbonyls. The component without hydrogens is the electrophile: O O O OH O C CH C C CH CH3 C H CH -H2O CH 2 CH3 H 3 ketone enolate no -hydrogens -unsaturated ketone Aldol Cyclizations- A dicarbonyl produces an enolate and carbonyl in the same molecule: enolate from a O OH 1,5-diketone CH OH 3 CH3 O O -H2O O CH2 CH3 CH3 CH2 O Claisen Condensation- Similar to Aldol condensation except the nucleophile is an ester enolate; O O O O O O + EtO C CH C OEt EtO C CH2 C EtO C CH2 CH3 C OEt 2 ketoester CH3 CH3 Dieckmann Cyclization- Internal Claisen condensation similar to Aldol cyclization. A 1,6 diester gives a 5-membered ring and a 1,7 diester gives a 6-membered ring: O OEt O OEt cyclic ketoester C C OEt O O Crossed Claisen- Similar to crossed Aldol- Electrophile has nohydrogens: O O O O C C EtO EtO C CH2 EtO C CH2 ketoester Variations on Crossed Claisen- ketone enolate and ester condensation. Esters, carbonates, formates and oxalates are common electrophiles: O O O O O O O O H C OEt H EtO C OEt OEt ethyl formate -ketoaldehyde diethyl carbonate -ketoester O O O O O OEt diketoester EtO C C OEt O diethyl oxalate -

SOM KINETIC STUDIES OH KETONE FORMATION THESIS Submitted for the Degree of Doctor of Philosophy at Glasgow University Hy Margare
S O M KINETIC STUDIES OH KETONE FORMATION THESIS submitted for the Degree of Doctor of Philosophy at Glasgow University hy Margaret Bennett Thornley May, 1956 Supervisor Dr. R. I. Reed. ProQuest Number: 13848948 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 13848948 Published by ProQuest LLC(2019). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 4 8 1 0 6 - 1346 TABLE OF CONTENTS. Page. SUMMARY. 2 Part I. HISTORICAL INTRODUCTION. 5 EXPERIMENTAL RESULTS. 13 Comparison of Reaction Rates in Different Media. 8® THEORETICAL INTERPRETATION OP RESULTS. S3 KINETIC OBSERVATIONS. 38 Dilatometry. 35 Colorimetric Estimation. 39 Chemical Analysis. 40 Experimental Determination. 43 Specimen Results. 44 Dieckmann Ring Closure of Diethyl Pimelate. 46 FORMAL PROOF OF UNIQUE RING FORMATION. 50 Experimental Procedure. 53 PREPARATIVE WORK. 55 Part II. HISTORICAL SURVEY. 59 PRELIMINARY EXPERIMENTAL WORK. 69 Preparation and Analysis of Sodium Salts. 71 KINETIC OBSERVATIONS. 74 Experimental Method. 75 Specimen Results. 76 EXPERIMENTAL RESULTS. 81 Page. EXPERIMENTAL RESULTS. 81 Mathematical Analysis. 82 Effect of Nature of Reaction Vessel. 86 To Investigate the Effect of Reaction Products. 89 Pyrolysis of Disodium (3-methyladipate. -
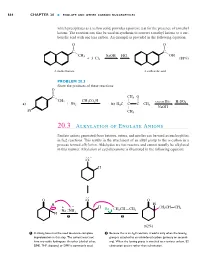
20.3 Alkylation of Enolate Anions
Hornback_Ch20_858-917 12/16/04 12:05 PM Page 864 864 CHAPTER 20 ■ ENOLATE AND OTHER CARBON NUCLEOPHILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone. The reaction can also be used in synthesis to convert a methyl ketone to a car- boxylic acid with one less carbon. An example is provided in the following equation: O O X X C– C– CH3 NaOH HCl OH ϩ 3 Cl2 (88%) A methyl ketone A carboxylic acid PROBLEM 20.3 Show the products of these reactions: O X C– CH3 O CH CH CO H W X 3 ϩ 3 2 – – excess Br2 H2SO4 a) Br2 b) H3C C C CH3 W NaOH Br CH3 20.3 Alkylation of Enolate Anions Enolate anions generated from ketones, esters, and nitriles can be used as nucleophiles in SN2 reactions. This results in the attachment of an alkyl group to the ␣-carbon in a process termed alkylation. Aldehydes are too reactive and cannot usually be alkylated in this manner. Alkylation of cyclohexanone is illustrated in the following equation: . – .O. H . O .O O H – H . œ + – H – œ CH2CH CH2 Na . NH Br CH2CH CH2 H 2 1 2 (62%) 1 A strong base must be used to ensure complete 2 Because this is an SN2 reaction, it works only when the leaving deprotonation in this step. The solvent must not group is attached to an unhindered carbon (primary or second- have any acidic hydrogens. An ether (diethyl ether, ary). When the leaving group is attached to a tertiary carbon, E2 DME, THF, dioxane) or DMF is commonly used. -

Aldol Condensation
Chemistry 212 Laboratory Dibenzalacetone via Crossed Aldol Condensation Prelab: Calculate the amounts of all chemicals needed in measurable amounts (i.e. grams or milliliters rather than moles.) Introduction: Aldol condensations are important in organic synthesis, providing a good way to form carbon–carbon bonds. The "aldol" (aldehyde + alcohol) product is a structural unit found in many naturally occurring molecules and pharmaceuticals, and is therefore important. In an Aldol condensation an enolate ion reacts with a carbonyl compound to form a β- hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give a conjugated enone. The general equation is shown in Figure 1. O O O R" B: H R R'" R "R R'" loss of H2O H R' R' Figure 1. The equation for the Aldol Condensation. The reaction involves the nucleophilic addition of an enolate to an aldehyde to form a β-hydroxy carbonyl. The β-hydroxy carbonyl is readily dehydrated under mild conditions. The aldol reaction occurs under both acidic and basic conditions as seen in Figure 2. ENOL pathway (reacts in H O protonated OH form) O O catalytic H+ O O H H R' H2O lost R' R R R' R R H aldol addition product aldol condensation product ENOLATE pathway O O M O M O base O H R' R R' R R enolate H Figure 2. The Aldol reaction and subsequent dehydration under acidic and basic conditions. The reaction we will be doing this week involves the reaction between benzaldehyde and acetone to do a double Aldol Condensation. The overall equation is shown in Figure 3. -

Biocatalyzed Synthesis of Statins: a Sustainable Strategy for the Preparation of Valuable Drugs
catalysts Review Biocatalyzed Synthesis of Statins: A Sustainable Strategy for the Preparation of Valuable Drugs Pilar Hoyos 1, Vittorio Pace 2 and Andrés R. Alcántara 1,* 1 Department of Chemistry in Pharmaceutical Sciences, Faculty of Pharmacy, Complutense University of Madrid, Campus de Moncloa, E-28040 Madrid, Spain; [email protected] 2 Department of Pharmaceutical Chemistry, Faculty of Life Sciences, Althanstrasse 14, A-1090 Vienna, Austria; [email protected] * Correspondence: [email protected]; Tel.: +34-91-394-1823 Received: 25 February 2019; Accepted: 9 March 2019; Published: 14 March 2019 Abstract: Statins, inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, are the largest selling class of drugs prescribed for the pharmacological treatment of hypercholesterolemia and dyslipidaemia. Statins also possess other therapeutic effects, called pleiotropic, because the blockade of the conversion of HMG-CoA to (R)-mevalonate produces a concomitant inhibition of the biosynthesis of numerous isoprenoid metabolites (e.g., geranylgeranyl pyrophosphate (GGPP) or farnesyl pyrophosphate (FPP)). Thus, the prenylation of several cell signalling proteins (small GTPase family members: Ras, Rac, and Rho) is hampered, so that these molecular switches, controlling multiple pathways and cell functions (maintenance of cell shape, motility, factor secretion, differentiation, and proliferation) are regulated, leading to beneficial effects in cardiovascular health, regulation of the immune system, anti-inflammatory and immunosuppressive properties, prevention and treatment of sepsis, treatment of autoimmune diseases, osteoporosis, kidney and neurological disorders, or even in cancer therapy. Thus, there is a growing interest in developing more sustainable protocols for preparation of statins, and the introduction of biocatalyzed steps into the synthetic pathways is highly advantageous—synthetic routes are conducted under mild reaction conditions, at ambient temperature, and can use water as a reaction medium in many cases. -

Art-Of-Drugs-Synthesis.Pdf
THE ART OF DRUG SYNTHESIS THE ART OF DRUG SYNTHESIS Edited by Douglas S. Johnson Jie Jack Li Pfizer Global Research and Development Copyright # 2007 by John Wiley & Sons, Inc. All rights reserved. Published by John Wiley & Sons, Inc., Hoboken, New Jersey Published simultaneously in Canada No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning, or otherwise, except as permitted under Section 107 or 108 of the 1976 United States Copyright Act, without either the prior written permission of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, Inc., 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 750-4470, or on the web at www.copyright.com. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, or online at http://www.wiley.com/go/permission. Limit of Liability/Disclaimer of Warranty: While the publisher and author have used their best efforts in preparing this book, they make no representations or warranties with respect to the accuracy or completeness of the contents of this book and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. No warranty may be created or extended by sales representatives or written sales materials. The advice and strategies contained herein may not be suitable for your situation. You should consult with a professional where appropriate. -

The Claisen Condensation
Lecture 19 The Claisen Condensation •• •• • • O • O • – • CH3COCH2CH3 • CH2 COCH2CH3 March 29, 2016 Chemistry 328N Exam Tomorrow Evening!! Review tonight 5PM -6PM Welch 1.316 Chemistry 328N Some “loose ends” before we go on Spectrosopy of acid derivatives A selective reduction for your tool box Chemistry 328N Reduction of Acid Derivatives Acids (page 679-681) Esters (page 738-739) Please work through the example on 738 Amides (page 739-742) Nitriles (page 742) Selective reductions with NaBH4 Chemistry 328N DIBAlH Diisobutylaluminum hydride (DIBAlH) at -78°C selectively reduces esters to aldehydes •at -78°C, the tetrahedral intermediate does not collapse and it is not until hydrolysis in aqueous acid that the carbonyl group of the aldehyde is liberated Stable at low temperature Chemistry 328N Infrared Spectroscopy C=O stretching frequency depends on whether the compound is an acyl chloride, anhydride, ester, or amide. C=O stretching frequency n O O O O O CH3CCl CH3COCCH3 CH3COCH3 CH3CNH2 1822 cm-1 1748 1736 cm-1 1694 cm-1 and 1815 cm-1 Chemistry 328N Infrared Spectroscopy Anhydrides have two peaks due to C=O stretching. One from symmetrical stretching of the C=O and the other from an antisymmetrical stretch. C=O stretching frequency n O O CH3COCCH3 1748 and 1815 cm-1 Chemistry 328N Infrared Spectroscopy Nitriles are readily identified by absorption due to carbon-nitrogen triple bond stretching that is “all alone” in the 2210-2260 cm-1 region. Chemistry 328N Hydrolysis and Decarboxylation Chemistry 328N t-Butyl esters Chemistry 328N t-Butyl esters Chemistry 328N t-Butyl ester hydrolysis Note which bond is broken in this hydrolysis !! Chemistry 328N Recall our discussion of the acidity of protons a to carbonyls The anion is stabilized by resonance The better the stabilization, the more acidic the a proton Acidity of a protons on“normal” aldehydes and ketones is about that of alcohols and less than water…pKa ~ 18-20 Some are far more acidic, i.e. -

The Claisen Condensation Is a Carbon–Carbon Bond Forming Reaction That Occurs Between Two Esters Or One Ester and Another Carb
Claisen Ester Condensation The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone. Requirements ❖ At least one of the reagents must be enolizable (have an α-proton and be able to undergo deprotonation to form the enolate anion). ❖ There are a number of different combinations of enolizable and nonenolizable carbonyl compounds that form a few different types of Claisen. ❖ The base used must not interfere with the reaction by undergoing nucleophilic substitution or addition with a carbonyl carbon. ❖ For this reason, the conjugate sodium alkoxide base of the alcohol formed (e.g. sodium ethoxide if ethanol is formed) is often used, since the alkoxide is regenerated. ❖ In mixed Claisen condensations, a non-nucleophilic base such as lithium diisopropylamide, or LDA, may be used, since only one compound is enolizable. ❖ LDA is not commonly used in the classic Claisen or Dieckmann condensations due to enolization of the electrophilic ester. ❖ The alkoxy portion of the ester must be a relatively good leaving group. ❖ Methyl and ethyl esters, which yields methoxide and ethoxide, respectively, are commonly used. Types Mechanism ❖ In the first step of the mechanism, an α-proton is removed by a strong base, resulting in the formation of an enolate anion, which is made relatively stable by the delocalization of electrons. ❖ Next, the carbonyl carbon of the (other) ester is nucleophilically attacked by the enolate anion. ❖ The alkoxy group is then eliminated (resulting in (re)generation of the alkoxide), and the alkoxide removes the newly formed doubly α-proton to form a new, highly resonance-stabilized enolate anion.