An Adaptive Explanation for the Horse-Like Shape of Seahorses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Diversity of Fish (Pisces) in Freshwater
Hydrobiologia (2008) 595:545–567 DOI 10.1007/s10750-007-9034-0 FRESHWATER ANIMAL DIVERSITY ASSESSMENT Global diversity of fish (Pisces) in freshwater C. Le´veˆque Æ T. Oberdorff Æ D. Paugy Æ M. L. J. Stiassny Æ P. A. Tedesco Ó Springer Science+Business Media B.V. 2007 Abstract The precise number of extant fish spe- species live in lakes and rivers that cover only 1% cies remains to be determined. About 28,900 species of the earth’s surface, while the remaining 16,000 were listed in FishBase in 2005, but some experts species live in salt water covering a full 70%. While feel that the final total may be considerably higher. freshwater species belong to some 170 families (or Freshwater fishes comprise until now almost 13,000 207 if peripheral species are also considered), the species (and 2,513 genera) (including only fresh- bulk of species occur in a relatively few groups: water and strictly peripheral species), or about the Characiformes, Cypriniformes, Siluriformes, 15,000 if all species occurring from fresh to and Gymnotiformes, the Perciformes (noteably the brackishwaters are included. Noteworthy is the fact family Cichlidae), and the Cyprinodontiformes. that the estimated 13,000 strictly freshwater fish Biogeographically the distribution of strictly fresh- water species and genera are, respectively 4,035 species (705 genera) in the Neotropical region, 2,938 (390 genera) in the Afrotropical, 2,345 (440 Guest editors: E. V. Balian, C. Le´veˆque, H. Segers & K. Martens genera) in the Oriental, 1,844 (380 genera) in the Freshwater Animal Diversity Assessment Palaearctic, 1,411 (298 genera) in the Nearctic, and 261 (94 genera) in the Australian. -

Article Evolutionary Dynamics of the OR Gene Repertoire in Teleost Fishes
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.09.434524; this version posted March 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Article Evolutionary dynamics of the OR gene repertoire in teleost fishes: evidence of an association with changes in olfactory epithelium shape Maxime Policarpo1, Katherine E Bemis2, James C Tyler3, Cushla J Metcalfe4, Patrick Laurenti5, Jean-Christophe Sandoz1, Sylvie Rétaux6 and Didier Casane*,1,7 1 Université Paris-Saclay, CNRS, IRD, UMR Évolution, Génomes, Comportement et Écologie, 91198, Gif-sur-Yvette, France. 2 NOAA National Systematics Laboratory, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560, U.S.A. 3Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, D.C., 20560, U.S.A. 4 Independent Researcher, PO Box 21, Nambour QLD 4560, Australia. 5 Université de Paris, Laboratoire Interdisciplinaire des Energies de Demain, Paris, France 6 Université Paris-Saclay, CNRS, Institut des Neurosciences Paris-Saclay, 91190, Gif-sur- Yvette, France. 7 Université de Paris, UFR Sciences du Vivant, F-75013 Paris, France. * Corresponding author: e-mail: [email protected]. !1 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.09.434524; this version posted March 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Teleost fishes perceive their environment through a range of sensory modalities, among which olfaction often plays an important role. -

Some Questions Concerning the Syngnathidae Brood Pouch
BULLETIN OF MARINE SCIENCE, 49(3): 741-747,1991 SOME QUESTIONS CONCERNING THE SYNGNATHIDAE BROOD POUCH Marie Y. Azzarello ABSTRACT For more than a century the physiological role of the Syngnathidae brood pouch has been the subject of scientific interest and debate. Some of the earliest investigators purported that the highly vascular brood pouch was physiologically adapted for the reception of fertilized eggs and for the sustenance of the embryos (i.e., a "pseudo-placenta"). Others posited that the brood pouch served as an osmoregulatory organ for the developing embryos. To determine whether the primary physiological role of the brood pouch is one of nutrition or osmoreg- ulation, Syngnathus scove/li embryos were removed from the brood pouch at different de- velopmental stages (4.0-13.0 mm TL), placed in sterilized, aerated, artificial seawater hyper- or iso-osmotic to the blood and pouch fluid, to which no nutritive substances were added. In hyperosmotic media 25.7% of the in vitro embryos completed their normal gestation versus 18.7% in iso-osmotic media. These results appear to indicate that the male Syng- nathidae brood pouch serves neither as the primary nutritional source nor as an osmotic buffer for the developing embryos after a length of 4.0 mm TL is achieved. Members of the Syngnathidae manifest atypical reproductive behavior and parental care. Reproduction is ovoviviparous with a complete reversal of the usual maternal and paternal brooding roles. Large telolecithal eggs produced by the female are fertilized by the male the moment they are deposited in his brood pouch. Embryos are then incubated throughout their entire gestation period, which varies depending upon genus and species, in the paternal brood pouch (Gill, 1905; Hubbs, 1943; Breder and Rosen, 1966). -

Bony Fish Guide
This guide will help you to complete the Bony Fish Observation Worksheet. Bony Fish Guide Fish (n.) An ectothermic (cold-blooded) vertebrate (with a backbone) aquatic (lives in water) animal that moves with the help of fins (limbs with no fingers or toes) and breathes with gills. This definition might seem very broad, and that is because fish are one of the most diverse groups of animals on the planet—there are a lot of fish in the sea (not to mention rivers, lakes and ponds). In fact, scientists count at least 32,000 species of fish—more than any other type of vertebrate. Fish are split into three broad classes: Jawless Fish Cartilaginous Fish Bony Fish (hagfish, lampreys, etc.) (sharks, rays, skates, etc.) (all other fish) This guide will focus on the Bony Fish. There are at least 28,000 species of bony fish, and they are found in almost every naturally occurring body of water on the planet. Bony fish range in size: • Largest: ocean sunfish (Mola mola), 11 feet, over 5,000 pounds • Smallest: dwarf pygmy goby (Pandaka pygmaea), ½ inch, a fraction of an ounce (This image is life size.) The following guide will help you learn more about the bony fish you can find throughout the New England Aquarium. Much of the guide is keyed to the Giant Ocean Tank, but can be applied to many kinds of fish. Even if you know nothing about fish, you can quickly learn a few things: The shape of a fish’s body, the position of its mouth and the shape of its tail can give you many clues as to its behavior and adaptations. -

Respiratory Disorders of Fish
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Disorders of the Respiratory System in Pet and Ornamental Fish a, b Helen E. Roberts, DVM *, Stephen A. Smith, DVM, PhD KEYWORDS Pet fish Ornamental fish Branchitis Gill Wet mount cytology Hypoxia Respiratory disorders Pathology Living in an aquatic environment where oxygen is in less supply and harder to extract than in a terrestrial one, fish have developed a respiratory system that is much more efficient than terrestrial vertebrates. The gills of fish are a unique organ system and serve several functions including respiration, osmoregulation, excretion of nitroge- nous wastes, and acid-base regulation.1 The gills are the primary site of oxygen exchange in fish and are in intimate contact with the aquatic environment. In most cases, the separation between the water and the tissues of the fish is only a few cell layers thick. Gills are a common target for assault by infectious and noninfectious disease processes.2 Nonlethal diagnostic biopsy of the gills can identify pathologic changes, provide samples for bacterial culture/identification/sensitivity testing, aid in fungal element identification, provide samples for viral testing, and provide parasitic organisms for identification.3–6 This diagnostic test is so important that it should be included as part of every diagnostic workup performed on a fish. -

THE FAMILY SYNGNATHIDAE (PISCES: SYNGNATHIFORMES) of Taiwanl
Bull. Inst. Zool., Academia Sinica 22(1): 67-82 (1983) THE FAMILY SYNGNATHIDAE (PISCES: SYNGNATHIFORMES) OF TAIWANl SIN-CHE LEE Institlile of Zoology, Academia Sinica, Taipei, Taiwan lI5t Republic of China (Received October 12, 1982) Sin-Che Lee (1983) The family Syngnathidae (Pisces: Syngnathiformes) of Tai wan. Bull. Inst. Zool., Academia Sinica 22(1): 67-82. A systematic review of the syn gnathid fishes found in the waters of Taiwan and its adjacent islands documents a total of 22 species in 16 genera. Among them,. Dunckerocampus dactyliophorus, Coelo notus liaspis, Halicampus koilomatodon, Microphis manadensis, Hippichthys heptagon us, H. spidfer, Syngnathus pelagicus, Corythoichthys f!avofasdatus, Solegnathus hardwickii, Haliichthys taeniophorous and Hippocampus erinaceus are new records for the Taiwan area. A family diagnosis. key to genera and species, brief synonyms, diagnosis, remarks and illustrations of each species are given. trimaculatus and both H. kelloggi and H. atteri The syngnathids including the pipefishes mus are synonyms of H. kuda. Solegnathus and seahorses are small fishes of tropical and guntheri and Syngnathus argyristictus are pro moderately warm temperate shallow coastal visionally removed from the list since no· data waters. Seahorses are more highly specialized are available. Thus only 8 valid species are than pipefishes but most seahorses are confined remained in Chen's list of Taiwan syngnathids. to marine waters and restricted to particular Neverthless, after a period of intensive collec habitat. On the other hand pipefishes have a tion the present author has found 13 addi wider distribution; they can tolerate greater fional species making a total 21 syngnathid temperature and salinity ranges. -

Reproductive Biology of the Opossum Pipefish, Microphis Brachyurus Lineatus, in Tecolutla Estuary, Veracruz, Mexico
Gulf and Caribbean Research Volume 16 Issue 1 January 2004 Reproductive Biology of the Opossum Pipefish, Microphis brachyurus lineatus, in Tecolutla Estuary, Veracruz, Mexico Martha Edith Miranda-Marure Universidad Nacional Autonoma de Mexico Jose Antonio Martinez-Perez Universidad Nacional Autonoma de Mexico Nancy J. Brown-Peterson University of Southern Mississippi, [email protected] Follow this and additional works at: https://aquila.usm.edu/gcr Part of the Marine Biology Commons Recommended Citation Miranda-Marure, M. E., J. A. Martinez-Perez and N. J. Brown-Peterson. 2004. Reproductive Biology of the Opossum Pipefish, Microphis brachyurus lineatus, in Tecolutla Estuary, Veracruz, Mexico. Gulf and Caribbean Research 16 (1): 101-108. Retrieved from https://aquila.usm.edu/gcr/vol16/iss1/17 DOI: https://doi.org/10.18785/gcr.1601.17 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf and Caribbean Research by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Gulf and Caribbean Research Vol 16, 101–108, 2004 Manuscript received September 25, 2003; accepted December 12, 2003 REPRODUCTIVE BIOLOGY OF THE OPOSSUM PIPEFISH, MICROPHIS BRACHYURUS LINEATUS, IN TECOLUTLA ESTUARY, VERACRUZ, MEXICO Martha Edith Miranda-Marure, José Antonio Martínez-Pérez, and Nancy J. Brown-Peterson1 Laboratorio de Zoología, Universidad Nacional Autónoma de México, Facultad de Estudios Superiores Iztacala. Av., de los Barrios No.1, Los Reyes Iztacala, Tlalnepantla, Estado de México, C.P. 05490 Mexico 1Department of Coastal Sciences, The University of Southern Mississippi, 703 East Beach Drive, Ocean Springs, MS 39564 USA ABSTRACT The reproductive biology of the opossum pipefish, Microphis brachyurus lineatus, was investigated in Tecolutla estuary, Veracruz, Mexico, to determine sex ratio, size at maturity, gonadal and brood pouch histology, reproductive seasonality, and fecundity of this little-known syngnathid. -

Order GASTEROSTEIFORMES PEGASIDAE Eurypegasus Draconis
click for previous page 2262 Bony Fishes Order GASTEROSTEIFORMES PEGASIDAE Seamoths (seadragons) by T.W. Pietsch and W.A. Palsson iagnostic characters: Small fishes (to 18 cm total length); body depressed, completely encased in Dfused dermal plates; tail encircled by 8 to 14 laterally articulating, or fused, bony rings. Nasal bones elongate, fused, forming a rostrum; mouth inferior. Gill opening restricted to a small hole on dorsolat- eral surface behind head. Spinous dorsal fin absent; soft dorsal and anal fins each with 5 rays, placed posteriorly on body. Caudal fin with 8 unbranched rays. Pectoral fins large, wing-like, inserted horizon- tally, composed of 9 to 19 unbranched, soft or spinous-soft rays; pectoral-fin rays interconnected by broad, transparent membranes. Pelvic fins thoracic, tentacle-like,withI spine and 2 or 3 unbranched soft rays. Colour: in life highly variable, apparently capable of rapid colour change to match substrata; head and body light to dark brown, olive-brown, reddish brown, or almost black, with dorsal and lateral surfaces usually darker than ventral surface; dorsal and lateral body surface often with fine, dark brown reticulations or mottled lines, sometimes with irregular white or yellow blotches; tail rings often encircled with dark brown bands; pectoral fins with broad white outer margin and small brown spots forming irregular, longitudinal bands; unpaired fins with small brown spots in irregular rows. dorsal view lateral view Habitat, biology, and fisheries: Benthic, found on sand, gravel, shell-rubble, or muddy bottoms. Collected incidentally by seine, trawl, dredge, or shrimp nets; postlarvae have been taken at surface lights at night. -

The Genome of the Gulf Pipefish Enables Understanding of Evolutionary Innovations C
Small et al. Genome Biology (2016) 17:258 DOI 10.1186/s13059-016-1126-6 RESEARCH Open Access The genome of the Gulf pipefish enables understanding of evolutionary innovations C. M. Small1†, S. Bassham1†, J. Catchen1,2†, A. Amores3, A. M. Fuiten1, R. S. Brown1,4, A. G. Jones5 and W. A. Cresko1* Abstract Background: Evolutionary origins of derived morphologies ultimately stem from changes in protein structure, gene regulation, and gene content. A well-assembled, annotated reference genome is a central resource for pursuing these molecular phenomena underlying phenotypic evolution. We explored the genome of the Gulf pipefish (Syngnathus scovelli), which belongs to family Syngnathidae (pipefishes, seahorses, and seadragons). These fishes have dramatically derived bodies and a remarkable novelty among vertebrates, the male brood pouch. Results: We produce a reference genome, condensed into chromosomes, for the Gulf pipefish. Gene losses and other changes have occurred in pipefish hox and dlx clusters and in the tbx and pitx gene families, candidate mechanisms for the evolution of syngnathid traits, including an elongated axis and the loss of ribs, pelvic fins, and teeth. We measure gene expression changes in pregnant versus non-pregnant brood pouch tissue and characterize the genomic organization of duplicated metalloprotease genes (patristacins) recruited into the function of this novel structure. Phylogenetic inference using ultraconserved sequences provides an alternative hypothesis for the relationship between orders Syngnathiformes and Scombriformes. Comparisons of chromosome structure among percomorphs show that chromosome number in a pipefish ancestor became reduced via chromosomal fusions. Conclusions: The collected findings from this first syngnathid reference genome open a window into the genomic underpinnings of highly derived morphologies, demonstrating that de novo production of high quality and useful reference genomes is within reach of even small research groups. -
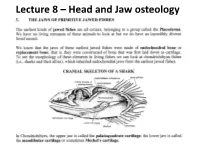
Lecture 8 – Head and Jaw Osteology
Lecture 8 – Head and Jaw osteology More derived fishes (Ray finned fishes) The variability of the jaw structure of bony fishes provides an explanation for the extensive adaptive radiation in the group and why they are so diverse and occupy almost every aquatic niche available. Skull diversity (A) carp, Cyprinus carpio, (B) vampire characin, Hydrolycus scomberoides, (C) catfish Arius felis. (D) cod Gadus morhua. (E) large-mouth bass, Micropterus salmoides (F) The parrotfish Scarus guacamaia. Scale bar = 10 mm WESTNEAT 2004 From an evolutionary standpoint, fishes were the first animals to develop bony jaws. Versatile jaws and multiple feeding strategies allowed fishes to fill, or radiate into, a diverse range of niches. They have evolved to feed in all possible ways – sucking, biting, scraping, nipping, crushing etc. The head of a teleost has 5 main regions: Cranium, jaws, cheeks, hydroid arch, opercula. The head of a fish has five main regions • 1) The CRANIUM is composed of the bones providing direct support and protection to the brain and the visual, Anterior Posterior olfactory, and auditory organs. Below the cranium is the parashenoid bone. • Parasphenoid plays a role in the jaws as Features of the neurocranium sensu lato (from Caranx it acts as a hard melampygus, lateral aspect, left, and posterior aspect, right). A = prevomer, B = ethmoid, C = frontal, D = palate supraoccipital, E = pterotic, F = exoccipital, G = basioccipital, H = foramen magnum, I = parasphenoid, J = orbit. The five main regions Bowfin 2) The JAWS • Lower Jaw – has an Angular articular and dentary bone • Angular articular- The paired bones form the posterior part of either side of the lower jaw and articulate with the suspensorium. -

Invasive Species of the Pacific Northwest
Invasive Species of the Pacific Northwest: Green Sunfish Lepomis cyanellus Derek Arterburn FISH 423: Olden 12.5.14 Figure 1: Adult Green sunfish Lepomis cyanellus . Photo from http://www.freshwater-fishing- news.com/fish-species-north -america/green-sunfish/ Classification Lepomis cyanellus may have a few teeth, Order: Perciformes which can be found on the tongue. Family: Centrarchidae Additional distinguishing marks are the 7-12 Genus: Lepomis parallel diffused dark bars running ventral to Species: cyanellus dorsal along the side of L. cyanellus, and the bluish-green pattern. The bluish-green Identification coloration takes place on the mainly black/dark brown/olive body, composed of Adult Green Sunfish, Lepomis ctenoid scales, which fades to a lighter cyanellus, commonly reach a total length of ventral color. The dark sides of L. cyanellus 31cm, with juveniles ranging from 12-15cm. are contrast with a yellow/cream ventral Adult Green Sunfish have been known to coloration (Cockerell 1913). The thick reach a maximum weight of one kilogram caudal peduncle is without an adipose fin, (2.2lbs). L. cyanellus is a deep bodied, and the peduncle runs to a rounded, slightly laterally compressed species, with a lateral forked, homocercal caudal fin. The paired line running from the operculum to the fins on Lepomis cyanellus are derived in caudal peduncle. The posterior of the orientation. The Green Sunfish has lateral operculum has a characteristic dark spot placement of the pectoral fins with vertical relatively the same size as the eye, and the insertion, anterior pelvic fins, and spines same size spot may also be found at the base found on the anal and dorsal fins. -

Acipenser Oxyrinchus) Ecological Risk Screening Summary
Atlantic sturgeon (Acipenser oxyrinchus) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, web version – 03/29/2018 Photo: Simon Pierre Barrette. Licensed under CC BY-SA 3.0. Available: https://commons.wikimedia.org/wiki/File:Acipenser_oxyrinchus_PAQ.jpg. 1 Native Range and Status in the United States Native Range From Froese and Pauly (2014): “Western Atlantic: Hamilton River, Labrador, Newfoundland, Canada to northeastern Florida, USA. Occurs occasionally in Bermuda and French Guiana [Robins and Ray 1986]. Northern Gulf of Mexico [Smith 1997]. In Europe: Baltic Sea. Landlocked populations in Lakes Ladoga and Onega (Russia), both now extirpated. Occasionally recorded from Great Britain and North Sea in Elbe drainage [Kottelat and Freyhof 2007]. Recent research revealed that this species existed in the Baltic Sea, but is now extirpated [Ludwig et al. 2002, 2008].” 1 Status in the United States From Froese and Pauly (2014): “USA: native” From NOAA Fisheries (2016): “Historically, Atlantic sturgeon were present in approximately 38 rivers in the United States from St. Croix, ME to the Saint Johns River, FL, of which 35 rivers have been confirmed to have had a historical spawning population. Atlantic sturgeon are currently present in approximately 32 of these rivers, and spawning occurs in at least 20 of them.” GBIF Secretariat (2014) contains a record in California that is assumed to be a misidentification as it is from 1956 and not substantiated elsewhere. Means of Introductions in the United States No records of non-native Acipenser oxyrinchus introductions in the United States were found. Remarks From Froese and Pauly (2014): “Near threatened globally, but extirpated in Europe [Kottelat and Freyhof 2007].