Materials Science
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Engineering Physics
COLLEGE OF ENGINEERING Engineering Physics WHY ENGINEERING PHYSICS? graduates have gone on to become the CEO Engineering is about the way things work. of Eastman Kodak Co., work as acoustical Physics is about why things work the way engineers for Disney, work for the CIA, thrive they do. By combining the two, engineering in the financial sector, work in nuclear physics students are able to satisfy their engineering at Seabrook Nuclear Power Plant, curiosity and ultimately gain a deeper manage the power flow for northern New understanding of the engineering problems England, work as radiation physicists at medical centers, and become high-level UM aine’s ADVANTAGE they are working to solve. executives at SanDisk and Avis. • Professors with Ph.D. degrees, not graduate WHY STUDY ENGINEERING PHYSICS AT UMAINE? RESEARCH OPPORTUNITIES students, teach classes UMaine engineering physics was the first UMaine engineering physics students are able • State-of-the-art teaching and accredited engineering physics program in to take advantage of a myriad of research research facilities the world. Currently, it is one of only 20 programs in the U.S. and the only opportunities as undergraduates — some as • Small, student-centered accredited one in New England. e early as their freshman year. Many work classes Department of Physics and Astronomy in closely with scientists in UMaine’s Laboratory for Surface Science and Technology, which is • Opportunities to do conjunction with the College of Engineering undergraduate research offers bachelor’s and master’s degrees in internationally known for cutting-edge alongside faculty engineering physics. e curriculum research with sensors. -
B.Sc. Mechatronics Specialization: Photonic Engineering
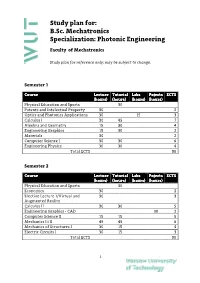
Study plan for: B.Sc. Mechatronics Specialization: Photonic Engineering Faculty of Mechatronics Study plan for reference only; may be subject to change. Semester 1 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 Patents and Intelectual Property 30 2 Optics and Photonics Applications 30 15 3 Calculus I 30 45 7 Algebra and Geometry 15 30 4 Engineering Graphics 15 30 2 Materials 30 2 Computer Science I 30 30 6 Engineering Physics 30 30 4 Total ECTS 30 Semester 2 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 Economics 30 2 Elective Lecture 1/Virtual and 30 3 Augmented Reality Calculus II 30 30 5 Engineering Graphics ‐ CAD 30 2 Computer Science II 15 15 5 Mechanics I i II 45 45 6 Mechanics of Structures I 30 15 4 Electric Circuits I 30 15 3 Total ECTS 30 1 Study Plan for B.Sc. Mechatronics (Spec. Photonic Engineering) Semester 3 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 0 Foreign Language 60 4 Elective Lecture 2/Introduction to 30 3 MEMS Calculus III 15 30 6 Mechanics of Structures II 15 15 4 Manufacturing Technology I 30 4 Fine Machine Design I 15 30 3 Electric Circuits II 30 3 Basics of Automation and Control I 30 15 4 Total ECTS 31 Semester 4 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 Foreign Language 60 4 Elective Lecture 3/Photographic 30 3 techniques in image acqusition Elective Lecture 4 30 3 /Enterpreneurship Optomechatronics 30 30 5 Electronics I 15 15 2 Electronics II 15 1 Fine Machine Design II 15 15 3 Manufacturing Technology 30 2 Metrology 30 30 4 Total ECTS 27 Semester 5 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 0 Foreign Language 60 4 Marketing 30 2 Elective Lecture 5/ Electric 30 2 2 Study Plan for B.Sc. -

Engineering Physics I Syllabus COURSE IDENTIFICATION Course
Engineering Physics I Syllabus COURSE IDENTIFICATION Course Prefix/Number PHYS 104 Course Title Engineering Physics I Division Applied Science Division Program Physics Credit Hours 4 credit hours Revision Date Fall 2010 Assessment Goal per Outcome(s) 70% INSTRUCTION CLASSIFICATION Academic COURSE DESCRIPTION This course is the first semester of a calculus-based physics course primarily intended for engineering and science majors. Course work includes studying forces and motion, and the properties of matter and heat. Topics will include motion in one, two, and three dimensions, mechanical equilibrium, momentum, energy, rotational motion and dynamics, periodic motion, and conservation laws. The laboratory (taken concurrently) presents exercises that are designed to reinforce the concepts presented and discussed during the lectures. PREREQUISITES AND/OR CO-RECQUISITES MATH 150 Analytic Geometry and Calculus I The engineering student should also be proficient in algebra and trigonometry. Concurrent with Phys. 140 Engineering Physics I Laboratory COURSE TEXT *The official list of textbooks and materials for this course are found on Inside NC. • COLLEGE PHYSICS, 2nd Ed. By Giambattista, Richardson, and Richardson, McGraw-Hill, 2007. • Additionally, the student must have a scientific calculator with trigonometric functions. COURSE OUTCOMES • To understand and be able to apply the principles of classical Newtonian mechanics. • To effectively communicate classical mechanics concepts and solutions to problems, both in written English and through mathematics. • To be able to apply critical thinking and problem solving skills in the application of classical mechanics. To demonstrate successfully accomplishing the course outcomes, the student should be able to: 1) Demonstrate knowledge of physical concepts by their application in problem solving. -

Engineering Physics I & Ii
ENGINEERING PHYSICS I & II DIPLOMA COURSE IN ENGINEERING FIRST AND SECOND SEMESTER A Publication under Government of Tamilnadu Distribution of Free Textbook Programme (NOT FOR SALE) Untouchability is a sin Untouchability is a crime Untouchability is a inhuman DIRECTORATE OF TECHNICAL EDUCATION GOVERNMENT OF TAMILNADU Government of Tamilnadu First Edition – 2015 THIRU. PRAVEEN KUMAR I.A.S Principal Secretary / Commissioner of Technical Education Directorate of Technical Education Guindy, Chennai- 600025 Dr. K.SUNDARAMOORTHY, M.E., Phd., Additional Director of Technical Education (Polytechnics) Directorate of Technical Education Guindy, Chennai- 600025 Co-ordinator Convener Er. R.SORNAKUMAR M.E., THIRU. K.SELVARAJAN M.Sc., Principal HOD (UG) / Physics Dr. Dharmambal Government Institute of Chemical Technology Polytechnic College for Women Tharamani, Chennai—113 Tharamani, Chennai—113 Reviewer Dr.K.SIVAKUMAR, M.Sc., Phd., Professor of Physics and Dean, Regional office Anna University, Madurai—7. Authors Dr.K.RAJESEKAR, M.Sc., Phd., Lecturer (UG)/ Physics Government Polytechnic College Nagercoil THIRU.G.MOHANA SUNDARAM, M.Sc.,M.Phil.,M.Ed., THIRU S.NAGARAJAN, M.Sc., HOD(UG)/Physics HOD(UG)/ Physics Central Polytechnic College Government Polytechnic College, Taramani, Chennai-113. Purasaiwakkam, Chennai –12 THIRU.N.SARAVANAN, M.Sc.,M.Phil,M.Ed., TMT.G.INDIRA, M.Sc.,M.Phil., HOD(UG)/Physics Lecturer/Physics Meenakshi Krishnan Polytechnic College Dr. Dharmambal Government Pammal, Chennai—75 Polytechnic College for Women Tharamani, Chennai—113 This book has been prepared by the Directorate of Technical Education This book has been printed on 60 G.S.M Paper Through the Tamil Nadu Text book and Educational Services Corporation ii FOREWORD With the concept of Global Village after liberalisation and globalisation, our country India has become one of the most sought after destinations by many Multi National Companies for investment. -

MSE 403: Ceramic Materials
MSE 403: Ceramic Materials Course description: Processing, characteristics, microstructure and properties of ceramic materials. Number of credits: 3 Course Coordinator: John McCloy Prerequisites by course: MSE 201 Prerequisites by topic: 1. Basic knowledge of thermodynamics. 2. Elementary crystallography and crystal structure. 3. Mechanical behavior of materials. Postrequisites: None Textbooks/other required 1. Carter, C.B. and Norton, M.G. Ceramic Materials Science and Engineering, materials: Springer, 2007. Course objectives: 1. Review of crystallography and crystal structure. 2. Review of structure of atoms, molecules and bonding in ceramics. 3. Discussion on structure of ceramics. 4. Effects of structure on physical properties. 5. Ceramic Phase diagrams. 6. Discussion on defects in ceramics. 7. Introduction to glass. 8. Discussion on processing of ceramics. 9. Introduction to sintering and grain growth. 10. Introduction to mechanical properties of ceramics. 11. Introduction to electrical properties of ceramics. 12. Introduction to bioceramics. 13. Introduction to magnetic ceramics. Topics covered: 1. Introduction to crystal structure and crystallography. 2. Fundamentals of structure of atoms. 3. Structure of ceramics and its influence on properties. 4. Binary and ternary phase diagrams. 5. Point defects in ceramics. 6. Glass and glass-ceramic composites. 7. Ceramics processing and sintering. 8. Mechanical properties of ceramics. 9. Electrical properties of ceramics. 10. Bio-ceramics. 11. Ceramic magnets. Expected student outcomes: 1. Knowledge of crystal structure of ceramics. 2. Knowledge of structure-property relationship in ceramics. 3. Knowledge of the defects in ceramics (Point defects). 4. Knowledge of glass and glass-ceramic composite materials. 5. Introductory knowledge on the processing of bulk ceramics. 6. Applications of ceramic materials in structural, biological and electrical components. -

Materials Science and Engineering 1
Materials Science and Engineering 1 EN.515.603. Materials Characterization. 3 Credits. MATERIALS SCIENCE AND This course will describe a variety of techniques used to characterize the structure and composition of engineering materials, including metals, ENGINEERING ceramics, polymers, composites, and semiconductors. The emphasis will be on microstructural characterization techniques, including optical The Materials Science and Engineering Program for professionals allows and electron microscopy, x-ray diffraction, and acoustic microscopy. students to take courses that address current and emerging areas Surface analytical techniques, including Auger electron spectroscopy, critical to the development and use of biomaterials, electronic materials, secondary ion mass spectroscopy, x-ray photoelectron spectroscopy, structural materials, nanomaterials and nanotechnology, and related and Rutherford backscattering spectroscopy. Real-world examples of materials processing technologies. Students in this program gain an materials characterization will be presented throughout the course, advanced understanding of foundational concepts and are exposed to including characterization of thin films, surfaces, interfaces, and single the latest research that is driving materials-related advances. crystals. Courses are offered at the Applied Physics Laboratory, the Homewood EN.515.605. Electrical, Optical and Magnetic Properties. 3 Credits. campus, and online. An overview of electrical, optical and magnetic properties arising from the fundamental electronic and atomic structure of materials. Continuum materials properties are developed through examination of microscopic Program Committee processes. Emphasis will be placed on both fundamental principles and James Spicer, Program Chair applications in contemporary materials technologies.Course Note(s): Principal Professional Staff Please note that this 515 course is also listed as a 510 course in the full- JHU Applied Physics Laboratory time program. -

Biological Materials: a Materials Science Approach✩
JOURNALOFTHEMECHANICALBEHAVIOROFBIOMEDICALMATERIALS ( ) ± available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/jmbbm Review article Biological materials: A materials science approachI Marc A. Meyers∗, PoYu Chen, Maria I. Lopez, Yasuaki Seki, Albert Y.M. Lin University of California, San Diego, La Jolla, CA, United States ARTICLEINFO ABSTRACT Article history: The approach used by Materials Science and Engineering is revealing new aspects Received 25 May 2010 in the structure and properties of biological materials. The integration of advanced Received in revised form characterization, mechanical testing, and modeling methods can rationalize heretofore 20 August 2010 unexplained aspects of these structures. As an illustration of the power of this Accepted 22 August 2010 methodology, we apply it to biomineralized shells, avian beaks and feathers, and fish scales. We also present a few selected bioinspired applications: Velcro, an Al2O3PMMA composite inspired by the abalone shell, and synthetic attachment devices inspired by gecko. ⃝c 2010 Elsevier Ltd. All rights reserved. Contents 1. Introduction and basic components ............................................................................................................................................. 1 2. Hierarchical nature of biological materials ................................................................................................................................... 3 3. Structural biological materials..................................................................................................................................................... -

Tennis Court Cleaner
Robotics Overview: The purpose of this session is to learn some basics about autonomous robots. What is a robot? A robot is a specialized kind of intelligent agent. Intelligent agents represent any kind of active decision-making behavior based on achieving some goal. Robots usually mean intelligent agents that are specifically designed to sense and adjust the “real world.” A good example of an intelligent agent that is not a robot would be a smart “physicians assistant” which helps diagnose patients and keeps track of multiple drug interactions. A good example of an intelligent agent that is a robot is the Martian explorer Opportunity. Figure 1: How robots, a type of intelligent agent, typically interact with the world. Figure 1 shows how most robots are designed to interact with the world, known as the feedback control loop. The field of robotics spans many disciplines essentially because of this diagram: sensors and actuators are built using knowledge of chemistry, physics, engineering, medicine and biology. Controls are built using knowledge of computer science, mathematics, and psychology. We will mostly worry about control, or the mind here. A primitive way to implement the idea of memory is to use a state machine. If you think about how you think (a subject of psychology and artificial intelligence) your mind often has these states: when hungry seek pizza, when sleepy seek bedroom, when board call friends, etc. A state diagram formalizes this kind of state-of-mind kind of thinking which helps to design robots to behave “intelligently.” State diagrams can have many states and ways to transition from one state to another. -

Materials Science
Materials Science Materials Science is an interdisciplinary field combining physics (fundamental laws of nature), chemistry (interactions of atoms) and biology (how life interacts with materials) to elucidate the inherent properties of basic and complex systems. This includes optical (interaction with light), electrical (interaction with charge), magnetic and structural properties of everyday electronics, clothing and architecture. The Materials Science central dogma follows the sequence: Structure—Properties—Design—Performance. This involves relating the nanostructure of a material to its macroscale physical and chemical properties. By understanding and then changing the structure, material scientists can create custom materials with unique properties. The goal of the materials science minor is to create a cross-disciplinary approach to fundamental topics in basic and applied physical sciences. Students will gain experience and perspectives from the disciplines of chemistry, physics and biology. The minor places a strong emphasis on current nanoscale research methods in addition to the basics of electronic, optical and mechanical properties of materials. Any student with an interest in pursuing the cross-disciplinary minor in materials science should consult with the coordinator of the minor. Students are encouraged to declare their participation in their sophomore year but no later than the end of the junior year. Students also should seek an adviser from participating faculty. Degree Requirements for the Minor General College requirements -

2. Electrochemistry
EP&M. Chemistry. Physical chemistry. Electrochemistry. 2. Electrochemistry. A phenomenon of electric current in solid conductors (class I conductors) is a flow of electrons caused by the electric field applied. For this reason, such conductors are also known as electronic conductors (metals and some forms of conducting non-metals, e.g., graphite). The same phenomenon in liquid conductors (class II conductors) is a flow of ions caused by the electric field applied. For this reason, such conductors are also known as ionic conductors (solutions of dissociated species and molten salts, known also as electrolytes). The question arises, what is the phenomenon causing the current flow across a boundary between the two types of conductors (known as an interface), i.e., when the circuit looks as in Fig.1. DC source Fig. 1. A schematic diagram of an electric circuit consis- flow of electrons ting of both electronic and ionic conductors. The phenomenon occuring at the interface must involve both the electrons and ions to ensure the continuity of the circuit. metal metal flow of ions ions containing solution When a conventional redox reaction occurs in a solution, the charge transfer (electron transfer) happens when the two reacting species get in touch with each other. For example: Ce4+ + Fe 2+→ Ce 3+ + Fe 3+ (2.1) In this reaction cerium (IV) draws an electron from iron (II) that leads to formation of cerium (III) and iron (III) ions. Cerium (IV), having a strong affinity for electrons, and therefore tending to extract them from other species, is called an oxidizing agent or an oxidant. -

Physical Chemistry MEHAU KULYK / SCIENCE PHOTO LIBRARY LIBRARY PHOTO SCIENCE / KULYK MEHAU
Physical chemistry MEHAU KULYK / SCIENCE PHOTO LIBRARY LIBRARY PHOTO SCIENCE / KULYK MEHAU 44 | Chemistry World | August 2010 www.chemistryworld.org Let’s get physical Physical chemists are finding themselves more in demand than ever. Emma Davies finds out why Physical chemistry is entering theory in harmony, he said: ‘with the called Fueling the future, his team something of a golden era. Its overall perspective of contributing In short is using IR spectroscopy to see tools have advanced dramatically accurate, experimentally vetted, The field of physical how protons are accommodated in recent years, so much so that molecular level pictures of reactive chemistry is booming, as in imidazole nanostructures. The scientists from all disciplines are pathways and relevant structures, more and more scientists project is based at the University of entering collaborations with physical physical chemists are in an excellent seek to understand their Massachussetts at Amhurst, US and chemists to gain new insight into position to engage chemistry in work on a molecular level teams are currently working on fuel their specialist subject areas. There is all of its complexity.’ He believes Developing alternative cells containing alternatives to nafion, however some worry that the subject that understanding processes at a energy sources is one a fluoropolymer-copolymer which is could become a victim of its own molecular level is crucial to making area benefiting from a good proton conductor but fails in success, with fundamental research grand scientific leaps forward. a physical chemistry meeting contemporary demand for losing out in the funding stakes to Johnson and his team are working approach high temperature operation. -

Syllabus Chem 646
SYLLABUS CHEM 646 Course title and number Physical Organic Chemistry, CHEM 646 Term Fall 2019 Meeting times and location MWF 10:20 am – 11:10 am, Room: CHEM 2121 Course Description and Learning Outcomes Prerequisites: Organic Chemistry I and II or equivalent undergraduate organic chemistry courses. Physical Organic Chemistry (CHEM646) is a graduate/senior-undergrad level course of advanced organic chemistry. Physical organic chemistry refers to a discipline of organic chemistry that focuses on the relationship between chemical structure and property/reactivity, in particular, applying experimental and theoretical tools of physical chemistry to the study of organic molecules and reactions. Specific focal points of study include the bonding and molecular orbital theory of organic molecules, stability of organic species, transition states, and reaction intermediates, rates of organic reactions, and non-covalent aspects of solvation and intermolecular interactions. CHEM646 is designed to prepare students for graduate research on broadly defined organic chemistry. This course will provide the students with theoretical and practical frameworks to understand how organic structures impact the properties of organic molecules and the mechanism for organic reactions. By the end of this course, students should be able to: 1. Gain in-depth understanding of the nature of covalent bonds and non-covalent interactions 2. Use molecule orbital theory to interpret the property and reactivity of organic species. 3. Understand the correlation between the structure and physical/chemical properties of organic molecules, such as stability, acidity, and solubility. 4. Predict the reactivity of organic molecules using thermodynamic and kinetic analyses 5. Probe the mechanism of organic reactions using theoretical and experimental approaches.