Deuterium-Depleted Water Influence on the Isotope
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Messengers from Outer Space
The complete absence of national laboratories for standardizing radiation measurements and the lack of physics departments in most radiotherapy centres in Latin America warrant the setting up of one or more regional dosimetry facilities, the functions of which would be primarily to calibrate dosimeters; to provide local technical assistance by means of trained staff; to check radiation equipment and dosimeters; to arrange a dose-intercomparison service; and to co-operate with local personnel dosimetry services. To be most effective, this activity should be in the charge of local personnel, with some initial assistance in the form of equipment and experts provided by the Agency. Although the recommendations were presented to the IAEA as the or ganization responsible for the setting up of the panel, the participants re commended that the co-operation of the World Health Organization and the Pan-American Health Organization should be invited. It was also suggested that the panel report be circulated to public health authorities in the countries represented. MESSENGERS FROM OUTER SPACE Although no evidence has yet been confirmed of living beings reaching the earth from space, it has been estimated that hundreds of tons of solid matter arrive every day in the form of meteorites or cosmic dust particles. Much is destroyed by heat in the atmosphere but fragments recovered can give valuable information about what has been happening in the universe for billions of years. Some of the results of worldwide research on meteorites were given at a symposium in Vienna during August. During six days of discussions a total of 73 scientific papers was present ed and a special meeting was held for the purpose of improving international co-operation in the research. -
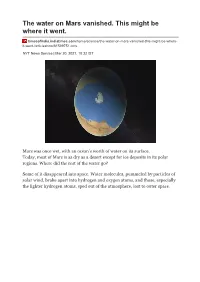
The Water on Mars Vanished. This Might Be Where It Went
The water on Mars vanished. This might be where it went. timesofindia.indiatimes.com/home/science/the-water-on-mars-vanished-this-might-be-where- it-went-/articleshow/81599751.cms NYT News Service | Mar 20, 2021, 10:32 IST Mars was once wet, with an ocean’s worth of water on its surface. Today, most of Mars is as dry as a desert except for ice deposits in its polar regions. Where did the rest of the water go? Some of it disappeared into space. Water molecules, pummeled by particles of solar wind, broke apart into hydrogen and oxygen atoms, and those, especially the lighter hydrogen atoms, sped out of the atmosphere, lost to outer space. A tall outcropping of rock, with layered deposits of sediments in the distance, marking a remnant of an ancient, long-vanished river delta in Jezero Crater, are pictured in this undated image taken by NASA's Mars rover Perseverance. (Reuters) But most of the water, a new study concludes, went down, sucked into the red planet’s rocks. And there it remains, trapped within minerals and salts. Indeed, as much as 99% of the water that once flowed on Mars could still be there, the researchers estimated in a paper published this week in the journal Science. Data from the past two decades of robotic missions to Mars, including NASA ’s Curiosity rover and the Mars Reconnaissance Orbiter, showed a wide distribution of what geologists call hydrated minerals. “It became very, very clear that it was common and not rare to find evidence of water alteration,” said Bethany Ehlmann, a professor of planetary science at the California Institute of Technology and one of the authors of the paper. -

The Protection of Frequencies for Radio Astronomy 1
JOURNAL OF RESEARCH of the National Bureau of Standards-D. Radio Propagation Vol. 67D, No. 2, March- April 1963 b The Protection of Frequencies for Radio Astronomy 1 R. 1. Smith-Rose President, International Scientific Radio Union (R eceived November 5, 1962) The International T elecommunications Union in its Geneva, 1959 R adio R egulations r recognises the Radio Astronomy Service in t he two following definitions: N o. 74 Radio A st1" onomy: Astronomy based on t he reception of waves of cos mi c origin. No. 75 R adio A st1"onomy Se1"vice: A service involving the use of radio astronomy. This service differs, however, from other r adio services in two important respects. 1. It does not itself originate any radio waves, and therefore causes no interference to any other service. L 2. A large proportion of its activity is conducted by the use of reception techniques which are several orders of magnit ude )]).ore sensitive than those used in other ra dio services. In order to pursue his scientific r esearch successfully, t he radio astronomer seeks pro tection from interference first, in a number of bands of frequencies distributed t hroughout I t he s p ~ct run:; and secondly:. 1~10r e complete and s p ec i~c prote.ction fOl: t he exact frequency bands III whIch natural radIatIOn from, or absorptIOn lD, cosmIc gases IS known or expected to occur. The International R egulations referred to above give an exclusive all ocation to one freq uency band only- the emission line of h ydrogen at 1400 to 1427 Mc/s. -

Effects of Moisture Content and Metabolic Water Production on Desert Rodent Seed Preferences
3V 'TOE EFFECTS OF MOISTURE CONTENT AND METABOLIC WATER PRODUCTION ON DESERT RODENT SEED PREFERENCES/ by Craig L. Frank A.S., Herkimer County Community College, 1981 B.S., State University of New York at Albany, 1984 A MASTER'S THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Biology KANSAS STATE UNIVERSITY Manhattan, Kansas 1987 Approved by: Major Professor Co-Major Professor . A112D7 3QflETfi TABLE OF CONTENTS PAGE Acknowledgments i I. Diet Optimization by a Heteromyid Rodent: The Role of Net Metabolic Water Production A. Abstract 2 B. Introduction 3 C Materials and Methods 9 D. Results 15 E. Discussion I3 F. Literature Cited 24 G. Tables 3I H. Figures 33 II. Diet Optimization by a Heteromyid Rodent: The Role of Moisture Content A. Abstract ^q B. Introduction ^2 C. Materials and Methods 43 D. Results 40 E. Discussion 52 F. Literature Cited 57 G. Tables ^3 H. Figures gc ^ General Abstract go ACKNOWLEDGMENTS I wish to first thank 0. J. Reichman and Christopher C. Smith who provided the inspiration and guidance necessary for this study. I also thank Donald P. Christian for helpful discussions and assistance. Donald W. Kaufman, John L. Zimmerman, and E.W. Evans provided critical comments on the manuscripts. Keith C. Behnke provived valuable assistance in producing the semi-synthetic diets. Jim Higgins assisted with the statistics. This study was supported by grants-in-aid of research from the American Society of Mammalogists and Sigma Xi. -1- I. DIET OPTIMIZATION BY A HETEROMYID RODENT: THE ROLE OF NET METABOLIC WATER PRODUCTION -1- ABSTRACT Kangaroo rats (family Heteromyidae) are primarily granivores. -

The Dole Effect and Its Variations During the Last 130,000 Years As Measured in the Vostok Ice Core
GLOBAL BIOGEOCHEMICAL CYCLES, VOL. 8, NO. 3, PAGES 363-376, SEPTEMBER 1994 The Dole effect and its variations during the last 130,000 years as measured in the Vostok ice core Michael Bender • and Todd Sowers 2 GraduateSchool of Oceanography,University of RhodeIsland, Kingston Laurent Labeyrie Centredes Faibles Radioactivites, Centre National de la RechercheScientifique, Commissariat a L'EnergieAtomique, Gif-sur- Yvette, France Abstract. We review the currentunderstanding of the Dole effect (the observeddifference between the •5180of atmospheric0 2 andthat of seawater)and its causes, extend the record of variationsin theDole effectback to 130kyr before present using data on the •5180 of 0 2obtained from studying the Vostok ice core(Sowers et al., 1993), anddiscuss the significanceof temporalvariations. The Dole effectreflects oxygenisotope fractionation during photosynthesis, respiration, and hydrologic processes (evaporation, precipitation,and evapotranspiration). Our bestprediction of the present-dayDole effect,+20.8 %0,is considerablylower thanthe observedvalue, + 23.5 %0,and we discusspossible causes of this discrepancy. During the past 130 kyr, the Dole effecthas been 0.05 %0lower thanthe present value, on average.The standarddeviation of the Dole effect from the meanhas been only _+0.2 %0,and the Dole effect is nearly unchangedbetween glacial maxima and interglacial periods. The smallvariability in theDole effect suggeststhat relative rates of primaryproduction in theland and marine realms have been relatively constant.Most periodicvariability in the Dole effectis in the precessionband, suggesting that changes in thisglobal biogeochemical term reflects variations in low-latitudeland hydrology and productivity or possiblyvariability in low-latitudeoceanic productivity. Introduction they can potentially be made to do so. Ice core reconstructionsof the concentrationsof bioactivegases in air Our understanding of the response of the biosphere to provide an integratedglobal signal which complementsthe Pleistoceneclimate change is actually quite limited. -

Deuterium – Tritium Pulse Propulsion with Hydrogen As Propellant and the Entire Space-Craft As a Gigavolt Capacitor for Ignition
Deuterium – Tritium pulse propulsion with hydrogen as propellant and the entire space-craft as a gigavolt capacitor for ignition. By F. Winterberg University of Nevada, Reno Abstract A deuterium-tritium (DT) nuclear pulse propulsion concept for fast interplanetary transport is proposed utilizing almost all the energy for thrust and without the need for a large radiator: 1. By letting the thermonuclear micro-explosion take place in the center of a liquid hydrogen sphere with the radius of the sphere large enough to slow down and absorb the neutrons of the DT fusion reaction, heating the hydrogen to a fully ionized plasma at a temperature of ~ 105 K. 2. By using the entire spacecraft as a magnetically insulated gigavolt capacitor, igniting the DT micro-explosion with an intense GeV ion beam discharging the gigavolt capacitor, possible if the space craft has the topology of a torus. 1. Introduction The idea to use the 80% of the neutron energy released in the DT fusion reaction for nuclear micro-bomb rocket propulsion, by surrounding the micro-explosion with a thick layer of liquid hydrogen heated up to 105 K thereby becoming part of the exhaust, was first proposed by the author in 1971 [1]. Unlike the Orion pusher plate concept, the fire ball of the fully ionized hydrogen plasma can here be reflected by a magnetic mirror. The 80% of the energy released into 14MeV neutrons cannot be reflected by a magnetic mirror for thermonuclear micro-bomb propulsion. This was the reason why for the Project Daedalus interstellar probe study of the British Interplanetary Society [2], the neutron poor deuterium-helium 3 (DHe3) reaction was chosen. -

What Are Cosmic Rays?!
WhatWWhatWhhaatt areaarearree CosmicCCosmicCoossmmiicc Rays?!RRays?!Raayyss??!! By Hayanon Translated by Y. Noda and Y. Kamide Supervised by Y. Muraki ᵶᵶᵋᵰᵿᶗᶑᵘᴾᴾᵱᶇᶀᶊᶇᶌᶅᶑᴾᶍᶄᴾᵡᶍᶑᶋᶇᶁᴾᵰᵿᶗᶑᵋᵰᵿᶗᶑᵘᵘᴾᴾᵱᶇᶀᶊᶊᶇᶌᶅᶑᴾᶍᶄᴾᵡᶍᶑᶋᶇᶁᴾᵰᵿᶗᶑ Have you ever had an X-ray examination Scientists identified three types at the hospital? In 1896, a German of radiation: positively-charged alpha physicist, W. C. Röntgen, astonished people particles, negatively-charged beta particles, with an image of bones captured through and uncharged gamma rays. In 1903, M. the use of X-rays. He had just discovered Curie along with her husband, P. Curie, and the new type of rays emitted by a discharge Becquerel, won the Nobel prize in physics. device. He named them X-rays. Because Furthermore, M. Curie was awarded the of their high penetration ability, they are Nobel prize in chemistry in 1911. able to pass through flesh. Soon after, it Certain types of radiation including was found that excessive use of X-rays can X-rays are now used for many medical cause harm to bodies. purposes including examining inside the In that same year, a French scientist, body, treating cancer, and more. Radiation, A. H. Becquerel, found that a uranium however, could be harmful unless the compound also gave off mysterious rays. amount of radiation exposure is strictly To his surprise, they could penetrate controlled. wrapping paper and expose a photographic The work with radium by M. Curie later film generating an image of the uranium led to the breakthrough discovery of compound. The uranium rays had similar the radiation coming from space. These characteristics as those of X-rays, but were cosmic rays were discovered by an Austrian determined to be different from them. -

Investigation of the Use of Deuterium and Oxygen in Illicit Fentanyl Analysis Author(S): John F
The author(s) shown below used Federal funding provided by the U.S. Department of Justice to prepare the following resource: Document Title: Investigation of the Use of Deuterium and Oxygen in Illicit Fentanyl Analysis Author(s): John F. Casale, Mike Lott, Jennifer R. Mallette Document Number: 255103 Date Received: August 2020 Award Number: NIJ-ONDCP IAA: 2017 DEA contract research task This resource has not been published by the U.S. Department of Justice. This resource is being made publically available through the Office of Justice Programs’ National Criminal Justice Reference Service. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Investigation of the Use of Deuterium and Oxygen in Illicit Fentanyl Analysis John F. Casalea, Mike Lottb, and Jennifer R. Mallettea aU.S. Drug Enforcement Administration Special Testing and Research Laboratory Dulles, VA bIsoForensics, Inc. Salt Lake City, UT 84108 This work was conducted through an Inter-Agency Agreement dated June 28, 2017, between the Office of National Drug Control Policy (ONDCP) and the National Institute of Justice (NIJ), under DEA Contract number 15DDHQ18P00000344 with funding from the NIJ, Office of Justice Programs, U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication/program/exhibition are those of the authors, and do not necessarily reflect those of the Department of Justice or ONDCP. This resource was prepared by the author(s) using Federal funds provided by the U.S. Department of Justice. -

Horaires Des Projections Screening Times
HORAIRES DES PROJECTIONS SCREENING TIMES LE POCKET GUIDE 2016 A ÉTÉ PUBLIÉ AVEC DES INFORMATIONS REÇUES JUSQU'AU 29/04/2016 THE POCKET GUIDE 2016 HAS BEEN EDITED WITH INFORMATION RECEIVED UP UNTIL 29/04/2016 Toutes les projections du Marché sont accessibles sur présentation du badge Marché du Film ou invitation délivrée par le Service Projections. Les projections dans les salles Lumière, Debussy, Buñuel, Salle du Soixantième, Théâtre Croisette et Miramar sont accessibles sur présentation du badge Marché ou Festival. Access to Marché du Film screenings is upon presentation of the Marché badge or an invitation given by the Marché Screening Department. Access to screenings taking place in Lumière, Debussy, Buñuel, Salle du Soixantième, Théâtre Croisette and Miramar is upon presentation of either a Marché or Festival badge. CO: Official Competition • HC: Out of Competition • CC: Cannes Classics • CR: Un Certain Regard • BLUE TITLE : Film screened for the first time at a market QR: Directors Fortnight • SC: Semaine de la critique • press: Press allowed • invite only: By invitation only • priority only: Priority badges only SUNDAY 15 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 08:30 (125’) CO 15:30 (158’) CO 19:30 (125’) CO 22:30 (116’) HC LUMIÈRE FROM THE LAND OF THE MOON AMERICAN HONEY FROM THE LAND OF THE MOON THE NICE GUYS TICKET REQUIRED Nicole Garcia Andrea Arnold Nicole Garcia Shane Black 2 294 seats STUDIOCANAL PROTAGONIST PICTURES STUDIOCANAL BLOOM 11:30 (145’) CO 14:30 (162’) CO 17:30 (120’) HC 19:45 (106’) HC SALLE AGASSI, THE -

Isotopic Fractionation of Carbon, Deuterium, and Nitrogen: a Full Chemical Study?
A&A 576, A99 (2015) Astronomy DOI: 10.1051/0004-6361/201425113 & c ESO 2015 Astrophysics Isotopic fractionation of carbon, deuterium, and nitrogen: a full chemical study? E. Roueff1;2, J. C. Loison3, and K. M. Hickson3 1 LERMA, Observatoire de Paris, PSL Research University, CNRS, UMR8112, Place Janssen, 92190 Meudon Cedex, France e-mail: [email protected] 2 Sorbonne Universités, UPMC Univ. Paris 6, 4 Place Jussieu, 75005 Paris, France 3 ISM, Université de Bordeaux – CNRS, UMR 5255, 351 cours de la Libération, 33405 Talence Cedex, France e-mail: [email protected] Received 6 October 2014 / Accepted 5 January 2015 ABSTRACT Context. The increased sensitivity and high spectral resolution of millimeter telescopes allow the detection of an increasing number of isotopically substituted molecules in the interstellar medium. The 14N/15N ratio is difficult to measure directly for molecules con- taining carbon. Aims. Using a time-dependent gas-phase chemical model, we check the underlying hypothesis that the 13C/12C ratio of nitriles and isonitriles is equal to the elemental value. Methods. We built a chemical network that contains D, 13C, and 15N molecular species after a careful check of the possible fraction- ation reactions at work in the gas phase. Results. Model results obtained for two different physical conditions that correspond to a moderately dense cloud in an early evolu- tionary stage and a dense, depleted prestellar core tend to show that ammonia and its singly deuterated form are somewhat enriched 15 14 15 + in N, which agrees with observations. The N/ N ratio in N2H is found to be close to the elemental value, in contrast to previous 15 + models that obtain a significant enrichment, because we found that the fractionation reaction between N and N2H has a barrier in + 15 + + 15 + the entrance channel. -

Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T
U.S. Department of the Interior U.S. Geological Survey Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T. B. Coplen', J. A. Hopple', J. K. Bohlke', H. S. Reiser', S. E. Rieder', H. R. o *< A R 1 fi Krouse , K. J. R. Rosman , T. Ding , R. D. Vocke, Jr., K. M. Revesz, A. Lamberty, P. Taylor, and P. De Bievre6 'U.S. Geological Survey, 431 National Center, Reston, Virginia 20192, USA 2The University of Calgary, Calgary, Alberta T2N 1N4, Canada 3Curtin University of Technology, Perth, Western Australia, 6001, Australia Institute of Mineral Deposits, Chinese Academy of Geological Sciences, Beijing, 100037, China National Institute of Standards and Technology, 100 Bureau Drive, Stop 8391, Gaithersburg, Maryland 20899 "institute for Reference Materials and Measurements, Commission of the European Communities Joint Research Centre, B-2440 Geel, Belgium Water-Resources Investigations Report 01-4222 Reston, Virginia 2002 U.S. Department of the Interior GALE A. NORTON, Secretary U.S. Geological Survey Charles G. Groat, Director The use of trade, brand, or product names in this report is for identification purposes only and does not imply endorsement by the U.S. Government. For additional information contact: Chief, Isotope Fractionation Project U.S. Geological Survey Mail Stop 431 - National Center Reston, Virginia 20192 Copies of this report can be purchased from: U.S. Geological Survey Branch of Information Services Box 25286 Denver, CO 80225-0286 CONTENTS Abstract...................................................... -
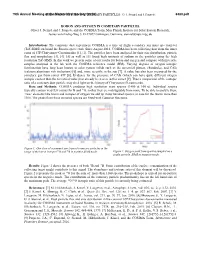
Boron and Oxygen in Cometary Particles: O. J
79th Annual Meeting ofBORON the Meteoritical AND OXYGEN Society IN COMETARY (2016) PARTICLES: O. J. Stenzel and J. Paquette 6380.pdf BORON AND OXYGEN IN COMETARY PARTICLES. Oliver J. Stenzel and J. Paquette and the COSIMA Team, Max Planck Institute for Solar System Research, Justus-von-Liebig-Weg 3, D-37077 Göttingen, Germany, [email protected]. Introduction: The cometary dust experiment COSIMA is a time of flight secondary ion mass spectrometer (ToF-SIMS) on board the Rosetta space craft. Since August 2014, COSIMA has been collecting dust from the inner coma of 67P/Churyumov-Gerasimenko [1], [2]. The particles have been analyzed for their size distribution, particle flux and morphology [3], [4]. [5] as well as [1] found high amounts of sodium in the particles using the high resolution ToF-SIMS. In this work we present some of our results for boron and oxygen and compare with meteorite samples analyzed in the lab with the COSIMA reference model (RM). Varying degrees of oxygen isotopic fractionation have long been known in solar system solids such as the terrestrial planets, chondrules, and CAIs (calcium-aluminum-rich inclusions) [6] and, more recently, in the sun [7]. A value has also been measured for the cometary gas from comet 67P [8]. Evidence for the presence of CAIs (which can have quite different oxygen isotopic content than the terrestrial value) has already been seen in this comet [9]. Thus a comparison of the isotopic ratio of a cometary dust particle may shed light on the history of Churyumov-Gerasimenko. Data and Methods: COSIMA produces high resolution mass spectra (1400 at 100 u).