End-Of-Line Hyphenation of Chemical Names (IUPAC Provisional
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unicode Nearly Plain-Text Encoding of Mathematics Murray Sargent III Office Authoring Services, Microsoft Corporation 4-Apr-06
Unicode Nearly Plain Text Encoding of Mathematics Unicode Nearly Plain-Text Encoding of Mathematics Murray Sargent III Office Authoring Services, Microsoft Corporation 4-Apr-06 1. Introduction ............................................................................................................ 2 2. Encoding Simple Math Expressions ...................................................................... 3 2.1 Fractions .......................................................................................................... 4 2.2 Subscripts and Superscripts........................................................................... 6 2.3 Use of the Blank (Space) Character ............................................................... 7 3. Encoding Other Math Expressions ........................................................................ 8 3.1 Delimiters ........................................................................................................ 8 3.2 Literal Operators ........................................................................................... 10 3.3 Prescripts and Above/Below Scripts........................................................... 11 3.4 n-ary Operators ............................................................................................. 11 3.5 Mathematical Functions ............................................................................... 12 3.6 Square Roots and Radicals ........................................................................... 13 3.7 Enclosures..................................................................................................... -

Chapter 2 Working with Text: Basics Copyright
Writer Guide Chapter 2 Working with Text: Basics Copyright This document is Copyright © 2021 by the LibreOffice Documentation Team. Contributors are listed below. You may distribute it and/or modify it under the terms of either the GNU General Public License (https://www.gnu.org/licenses/gpl.html), version 3 or later, or the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), version 4.0 or later. All trademarks within this guide belong to their legitimate owners. Contributors To this edition Rafael Lima Jean Hollis Weber Kees Kriek To previous editions Jean Hollis Weber Bruce Byfield Gillian Pollack Ron Faile Jr. John A. Smith Hazel Russman John M. Długosz Shravani Bellapukonda Kees Kriek Feedback Please direct any comments or suggestions about this document to the Documentation Team’s mailing list: [email protected] Note Everything you send to a mailing list, including your email address and any other personal information that is written in the message, is publicly archived and cannot be deleted. Publication date and software version Published April 2021. Based on LibreOffice 7.1 Community. Other versions of LibreOffice may differ in appearance and functionality. Using LibreOffice on macOS Some keystrokes and menu items are different on macOS from those used in Windows and Linux. The table below gives some common substitutions for the instructions in this document. For a detailed list, see the application Help. Windows or Linux macOS equivalent Effect Tools > Options LibreOffice > -

Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances 2017
INTERNATIONAL NARCOTICS CONTROL BOARD Precursors and chemicals frequently used in the illicit manufacture of narcotic drugs and psychotropic substances 2017 EMBARGO Observe release date: Not to be published or broadcast before Thursday, 1 March 2018, at 1100 hours (CET) UNITED NATIONS CAUTION Reports published by the International Narcotics Control Board in 2017 The Report of the International Narcotics Control Board for 2017 (E/INCB/2017/1) is supplemented by the following reports: Narcotic Drugs: Estimated World Requirements for 2018—Statistics for 2016 (E/INCB/2017/2) Psychotropic Substances: Statistics for 2016—Assessments of Annual Medical and Scientific Requirements for Substances in Schedules II, III and IV of the Convention on Psychotropic Substances of 1971 (E/INCB/2017/3) Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances: Report of the International Narcotics Control Board for 2017 on the Implementation of Article 12 of the United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988 (E/INCB/2017/4) The updated lists of substances under international control, comprising narcotic drugs, psychotropic substances and substances frequently used in the illicit manufacture of narcotic drugs and psychotropic substances, are contained in the latest editions of the annexes to the statistical forms (“Yellow List”, “Green List” and “Red List”), which are also issued by the Board. Contacting the International Narcotics Control Board The secretariat of the Board may be reached at the following address: Vienna International Centre Room E-1339 P.O. Box 500 1400 Vienna Austria In addition, the following may be used to contact the secretariat: Telephone: (+43-1) 26060 Fax: (+43-1) 26060-5867 or 26060-5868 Email: [email protected] The text of the present report is also available on the website of the Board (www.incb.org). -

Community College of Denver's Style Guide for Web and Print Publications
Community College of Denver’s Style Guide for Web and Print Publications CCD’s Style Guide supplies all CCD employees with one common goal: to create a functioning, active, and up-to-date publications with universal and consistent styling, grammar, and punctuation use. About the College-Wide Editorial Style Guide The following strategies are intended to enhance consistency and accuracy in the written communications of CCD, with particular attention to local peculiarities and frequently asked questions. For additional guidelines on the mechanics of written communication, see The AP Style Guide. If you have a question about this style guide, please contact the director of marketing and communication. Web Style Guide Page 1 of 10 Updated 2019 Contents About the College-Wide Editorial Style Guide ............................................... 1 One-Page Quick Style Guide ...................................................................... 4 Building Names ............................................................................................................. 4 Emails .......................................................................................................................... 4 Phone Numbers ............................................................................................................. 4 Academic Terms ............................................................................................................ 4 Times .......................................................................................................................... -

User Name Character Restrictions
User Name Character Restrictions • Feature Summary and Revision History, page 1 • Feature Changes, page 2 Feature Summary and Revision History Summary Data Applicable Product(s) or Functional All Area Applicable Platform(s) All Feature Default Enabled - Always-on Related Changes in This Release Not Applicable Related Documentation • System Administration Guide Revision History Note Revision history details are not provided for features introduced before releases 21.2 and N5.5. Revision Details Release User names can now only contain alphanumeric characters (a-z, A-Z, 0-9), hyphen, 21.3 underscore, and period. The hyphen character cannot be the first character. Release Change Reference, StarOS Release 21.3/Ultra Services Platform Release N5.5 1 User Name Character Restrictions Feature Changes Feature Changes Previous Behavior: User names previously could be made up of any string, including spaces within quotations. New Behavior: With this release, AAA user names and local user names can only use alphanumeric characters (a-z, A-Z, 0-9), hyphen, underscore, and period. The hyphen character cannot be the first character. If you attempt to create a user name that does not adhere to these standards, you will receive the following message: "Invalid character; legal characters are "0123456789.-_abcdefghijklmnopqrstuvwxyzABCDEFGHIJKLMNOPQRSTUVWXYZ". Impact on Customer: Existing customers with user names configured with special characters must re-configure those impacted users before upgrading to 21.3. Otherwise, those users will no longer be able to login after the upgade as their user name is invalid and is removed from the user database. Release Change Reference, StarOS Release 21.3/Ultra Services Platform Release N5.5 2. -

The Stata Journal
The Stata Journal View metadata, citation and similar papers at core.ac.uk brought to you by CORE Editor Executive Editor provided by Research Papers in Economics H. Joseph Newton Nicholas J. Cox Department of Statistics Department of Geography Texas A & M University University of Durham College Station, Texas 77843 South Road 979-845-3142; FAX 979-845-3144 Durham City DH1 3LE UK [email protected] [email protected] Associate Editors Christopher Baum J. Scott Long Boston College Indiana University Rino Bellocco Thomas Lumley Karolinska Institutet University of Washington, Seattle David Clayton Roger Newson Cambridge Inst. for Medical Research King’s College, London Mario A. Cleves Marcello Pagano Univ. of Arkansas for Medical Sciences Harvard School of Public Health Charles Franklin Sophia Rabe-Hesketh University of Wisconsin, Madison University of California, Berkeley Joanne M. Garrett J. Patrick Royston University of North Carolina MRC Clinical Trials Unit, London Allan Gregory Philip Ryan Queen’s University University of Adelaide James Hardin Mark E. Schaffer University of South Carolina Heriot-Watt University, Edinburgh Stephen Jenkins Jeroen Weesie University of Essex Utrecht University Jens Lauritsen Jeffrey Wooldridge Odense University Hospital Michigan State University Stanley Lemeshow Ohio State University Stata Press Production Manager Lisa Gilmore Copyright Statement: The Stata Journal and the contents of the supporting files (programs, datasets, and help files) are copyright c by StataCorp LP. The contents of the supporting files (programs, datasets, and help files) may be copied or reproduced by any means whatsoever, in whole or in part, as long as any copy or reproduction includes attribution to both (1) the author and (2) the Stata Journal. -

Analysis of Ammonia and Volatile Organic Amine Emissions in a Confined Poultry Facility
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 5-2010 Analysis of Ammonia and Volatile Organic Amine Emissions in a Confined Poultry Facility Hanh Hong Thi Dinh Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Analytical Chemistry Commons Recommended Citation Dinh, Hanh Hong Thi, "Analysis of Ammonia and Volatile Organic Amine Emissions in a Confined Poultry Facility" (2010). All Graduate Theses and Dissertations. 598. https://digitalcommons.usu.edu/etd/598 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Copyright © Hanh Hong Thi Dinh 2010 All Right Reserved iii ABSTRACT Analysis of Ammonia and Volatile Organic Amine Emissions in a Confined Poultry Facility by Hanh Hong Thi Dinh, Master of Science Utah State University, 2010 Major Professor: Dr Robert S. Brown Department: Chemistry and Biochemistry The National Air Emission Monitoring Study (NAEMS) project was funded by the Agricultural Air Research Council (AARC) to evaluate agricultural emissions nationwide. Utah State University (USU) is conducting a parallel study on agricultural emissions at a Cache Valley poultry facility. As part of this parallel study, samples of animal feed, eggs and animal waste were collected weekly from three manure barns (designated: manure barn, barn 4 - manure belt and barn 5 - high rise) from May 2008 to November 2009. These samples were analyzed to determine ammonia content, total Kjeldahl nitrogen content and ammonia emission. -

Efficient Synthesis of a Chiral 2-Aryl Pyrrolidine
PROCESS DEVELOPMENT Peer reviewed Jared Piper Efficient synthesis of a chiral 2-aryl Pyrrolidine JARED L. PIPER1,*, STANLEY P. KOLIS1, CHAOYU XIE1, MARTIAL BERTRAND2, PASCAL BOQUEL2 *Corresponding author 1. Eli Lilly and Company, Lilly Corporate Centre, Chemical Product Research and Development, Indianapolis, IN 46285, USA 2. Eli Lilly & Company, Lilly Development Centre, Chemical Product Research and Development, S.A., Rue Granbonpré 11, Mont-Saint-Guibert, B-1348, Belgium KEYWORDS Asymmetric synthesis; pyrrolidine; iridium catalysts. ABSTRACT 2-aryl pyrrolidine 1 was efficiently prepared in three steps starting from N-vinylpyrrolidinone and ethyl 3,5-dimethylbenzoate. The key steps highlight the use of a rearrangement and an iridium catalysed hydrogenation to afford the desired compound. This methodology was utilised to prepare several differentially 2-aryl-substituted pyrrolidines in good yield and enantiomeric excess, and is complimentary to existing methods to prepare molecules of this type. 38 Scheme 1. First generation synthesis of 1. INTRODUCTION hemical compounds enantioselective reduction, the synthesis had potential limitations. containing the pyrrolidine The Grignard chemistry required long reaction times (>24 hours), Cstructural motif are was difficult to initiate, and failed to reach complete conversion on ubiquitous in nature, and are present larger scale. In addition, multiple impurities were observed for the in both the core of the amino acids reaction, and the rejection of these substances from the product proline and hydroxyproline, and the stream was not trivial. During the cyclization event to form 6, the natural alkaloids nicotine and ficine. retention of stereochemical integrity of 5 during the course of the These heterocycles have been used reaction was unpredictable. -
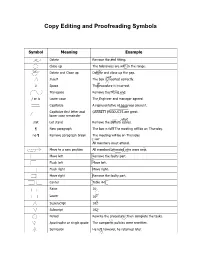
Copy Editing and Proofreading Symbols
Copy Editing and Proofreading Symbols Symbol Meaning Example Delete Remove the end fitting. Close up The tolerances are with in the range. Delete and Close up Deltete and close up the gap. not Insert The box is inserted correctly. # # Space Theprocedure is incorrect. Transpose Remove the fitting end. / or lc Lower case The Engineer and manager agreed. Capitalize A representative of nasa was present. Capitalize first letter and GARRETT PRODUCTS are great. lower case remainder stet stet Let stand Remove the battery cables. ¶ New paragraph The box is full. The meeting will be on Thursday. no ¶ Remove paragraph break The meeting will be on Thursday. no All members must attend. Move to a new position All members attended who were new. Move left Remove the faulty part. Flush left Move left. Flush right Move right. Move right Remove the faulty part. Center Table 4-1 Raise 162 Lower 162 Superscript 162 Subscript 162 . Period Rewrite the procedure. Then complete the tasks. ‘ ‘ Apostrophe or single quote The companys policies were rewritten. ; Semicolon He left however, he returned later. ; Symbol Meaning Example Colon There were three items nuts, bolts, and screws. : : , Comma Apply pressure to the first second and third bolts. , , -| Hyphen A valuable byproduct was created. sp Spell out The info was incorrect. sp Abbreviate The part was twelve feet long. || or = Align Personnel Facilities Equipment __________ Underscore The part was listed under Electrical. Run in with previous line He rewrote the pages and went home. Em dash It was the beginning so I thought. En dash The value is 120 408. -

Classifying Type Thunder Graphics Training • Type Workshop Typeface Groups
Classifying Type Thunder Graphics Training • Type Workshop Typeface Groups Cla sifying Type Typeface Groups The typefaces you choose can make or break a layout or design because they set the tone of the message.Choosing The the more right you font know for the about job is type, an important the better design your decision.type choices There will are be. so many different fonts available for the computer that it would be almost impossible to learn the names of every one. However, manys typefaces share similar qualities. Typographers classify fonts into groups to help Typographers classify type into groups to help remember the different kinds. Often, a font from within oneremember group can the be different substituted kinds. for Often, one nota font available from within to achieve one group the samecan be effect. substituted Different for anothertypographers usewhen different not available groupings. to achieve The classifi the samecation effect. system Different used by typographers Thunder Graphics use different includes groups. seven The major groups.classification system used byStevenson includes seven major groups. Use the Right arrow key to move to the next page. • Use the Left arrow key to move back a page. Use the key combination, Command (⌘) + Q to quit the presentation. Thunder Graphics Training • Type Workshop Typeface Groups ����������������������� ��������������������������������������������������������������������������������� ���������������������������������������������������������������������������� ������������������������������������������������������������������������������ -

The Not So Short Introduction to Latex2ε
The Not So Short Introduction to LATEX 2ε Or LATEX 2ε in 139 minutes by Tobias Oetiker Hubert Partl, Irene Hyna and Elisabeth Schlegl Version 4.20, May 31, 2006 ii Copyright ©1995-2005 Tobias Oetiker and Contributers. All rights reserved. This document is free; you can redistribute it and/or modify it under the terms of the GNU General Public License as published by the Free Software Foundation; either version 2 of the License, or (at your option) any later version. This document is distributed in the hope that it will be useful, but WITHOUT ANY WARRANTY; without even the implied warranty of MERCHANTABILITY or FITNESS FOR A PARTICULAR PURPOSE. See the GNU General Public License for more details. You should have received a copy of the GNU General Public License along with this document; if not, write to the Free Software Foundation, Inc., 675 Mass Ave, Cambridge, MA 02139, USA. Thank you! Much of the material used in this introduction comes from an Austrian introduction to LATEX 2.09 written in German by: Hubert Partl <[email protected]> Zentraler Informatikdienst der Universität für Bodenkultur Wien Irene Hyna <[email protected]> Bundesministerium für Wissenschaft und Forschung Wien Elisabeth Schlegl <noemail> in Graz If you are interested in the German document, you can find a version updated for LATEX 2ε by Jörg Knappen at CTAN:/tex-archive/info/lshort/german iv Thank you! The following individuals helped with corrections, suggestions and material to improve this paper. They put in a big effort to help me get this document into its present shape. -

19. Punctuation
punctuation 19. Punctuation Punctuation is important because it helps achieve clarity and readability . ’ Apostrophes Use • before the “s” in singular possessives: The prime minister’s suggestion was considered. • after the “s” in plural possessives: The ministers’ decision was unanimous. Do not use • in plural dates and abbreviations: 1930s, NGOs • in the possessive pronoun “its”: The government characterised its budget as prudent. See also: Capitalisation, pp. 66-68. : Colons Use • to lead into a list, an explanation or elaboration, an indented quotation • to mark the break between a title and subtitle: Social Sciences for a Digital World: Building Infrastructure for the Future (book) Trends in transport to 2050: A macroscopic view (chapter) Do not use • more than once in a given sentence • a space before colons and semicolons. 90 oecd style guide - third edition @oecd 2015 punctuation , Commas Use • to separate items in most lists (except as indicated under semicolons) • to set off a non-restrictive relative clause or other element that is not part of the main sentence: Mr Smith, the first chairperson of the committee, recommended a fully independent watchdog. • commas in pairs; be sure not to forget the second one • before a conjunction introducing an independent clause: It is one thing to know a gene’s chemical structure, but it is quite another to understand its actual function. • between adjectives if each modifies the noun alone and if you could insert the word “and”: The committee recommended swift, extensive changes. Do not use • after “i.e.” or “e.g.” • before parentheses • preceding and following en-dashes • before “and”, at the end of a sequence of items, unless one of the items includes another “and”: The doctor suggested an aspirin, half a grapefruit and a cup of broth.