Macropattern of Styloid and Druse Crystals in Quillaja (Quillajaceae) Bark and Leaves Nels R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GRAS Notice (GRN) No. 903, Quillaia Extract Type 2
GRAS Notice (GRN) No. 903 https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory NATUREX• part of Givaudan 'fR1@CG~nill~(jJ) 07 January 2020 JAN 1 5 2020 Dr. Paulette Gaynor FOOD OF, ,Li,; UF L ADDITIVE SAFETY Office of Food Additive Safety (HFS-200) __J Center for Food Safety and Applied Nutrition (CFSAN) Food and Drug Administration 5001 Campus Drive College Park, MD 20740 USA Dear Dr. Gaynor: Re: GRAS Notice for Qulllaia Extract Type 2 In accordance with 21 CFR §170 Subpart E consisting of§§ 170.203 through 170.285, Naturex SA (250 rue Pierre Bayle, BP 81218 - 84911, Avignon, Cedex 9, France], as the notifier, is submitting one hard copy and one electronic copy (on CD) of the GRAS Notice for quillaia extract type 2, containing all data and lnfonnation supporting the company's conclusion that quillaia extract type 2 is GRAS on the basis of scientific procedures, for use in specified conventional food and beverage products across multiple categories; these food uses of quillaia extract type 2 are therefore not subject to the premarket approval requirements of the Federal Food, Drug and Cosmetic Act. Information setting forth the basis for Naturex's GRAS conclusion, as well as a consensus opinion of an independent panel of experts, also are enclosed for review by the agency. I certify that the enclosed electronic files were scanned for viruses prior to submission and are thus certified as being virus-free using Symantec Endpoint Protection 12.1.4. Should you have any questions or concerns regarding this GRAS Notice, please do not hesitate to contact me at any point during the review process so that we may provide a response in a timely manner. -
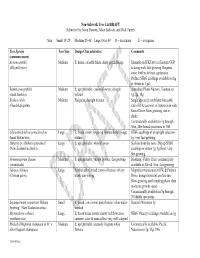
Recommended Non-Sidewalk Tree List DRAFT
Non-Sidewalk Tree List DRAFT Submitted by Jason Dewees, Mike Sullivan, and Dick Turner Size: Small 15-25’ Medium 25-40’ Large Over 40’ D = deciduous E = evergreen Tree Species Tree Size Design Characteristics Comments (common name) Acmena smithii Medium E; dense, colorful fruits; shiny green foliage Example on JFK Drive in Eastern GGP (lilly-pilly tree) is doing well; fast-growing. Requires some fertility for best appearance. Profuse SFBG seedlings available to dig or obtain in 1 gal. Banksia integrifolia Medium E; upright habit; creamy flowers; drought Australian Plants Nursery, Ventura, in (coast banksia) tolerant 1g, 5g, 15g Brahea edulis Medium Fan palm; drought tolerant Single specimen established on north (Guadalupe palm) side of JFK just west of intersection with Kezar Drive. Slow growing, sun or shade. Commercially available in 1g through 36in, 48in boxed specimens to 15ft Chiranthodendron pentadactylon Large E; broad crown; large red flowers, bold foliage SFBG seedlings of an upright selection: (hand flower tree) texture 1g; very fast-growing Hoheria sp. (Hoheria populnea? Large E; upright habit; white flowers Suckers from the roots. Dig up SFBG (New Zealand lacebark)) seedlings or obtain 1g, 5g there; very fast-growing Hymenosporum flavum Medium E; upright habit; yellow flowers; fast growing Boething, Valley Crest; commercially (sweetshade) available in 24in & 36in; fast-growing. Jubaea chilensis Large Feather palm; broad crown of leaves; silvery Magnificent specimen at JFK & Fuchsia (Chilean palm) trunk; sun-loving Drive; drought-tolerant, prefers sun. Slow-growing until trunking phase, then moderate growth speed. Commercially available in 5g through 20ft B&B specimens Leptospermum scoparium ‘Helene Small E; broad, low crown; pink flowers; often multi- Suncrest Nurseries 5g Strybing’ (New Zealand tea tree) trunked Metrosideros robusta Large E; broad dense crown; showy red flowers in SFBG Nursery seedlings available in 1g (northern rata) summer; slow & unusual but very well-adapted Michelia/Magnolia champaca or M. -

Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1
Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1 Authors: Jiang, Wei, He, Hua-Jie, Lu, Lu, Burgess, Kevin S., Wang, Hong, et. al. Source: Annals of the Missouri Botanical Garden, 104(2) : 171-229 Published By: Missouri Botanical Garden Press URL: https://doi.org/10.3417/2019337 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Downloaded From: https://bioone.org/journals/Annals-of-the-Missouri-Botanical-Garden on 01 Apr 2020 Terms of Use: https://bioone.org/terms-of-use Access provided by Kunming Institute of Botany, CAS Volume 104 Annals Number 2 of the R 2019 Missouri Botanical Garden EVOLUTION OF ANGIOSPERM Wei Jiang,2,3,7 Hua-Jie He,4,7 Lu Lu,2,5 POLLEN. 7. NITROGEN-FIXING Kevin S. Burgess,6 Hong Wang,2* and 2,4 CLADE1 De-Zhu Li * ABSTRACT Nitrogen-fixing symbiosis in root nodules is known in only 10 families, which are distributed among a clade of four orders and delimited as the nitrogen-fixing clade. -

Phylogeny of Maleae (Rosaceae) Based on Multiple Chloroplast Regions: Implications to Genera Circumscription
Hindawi BioMed Research International Volume 2018, Article ID 7627191, 10 pages https://doi.org/10.1155/2018/7627191 Research Article Phylogeny of Maleae (Rosaceae) Based on Multiple Chloroplast Regions: Implications to Genera Circumscription Jiahui Sun ,1,2 Shuo Shi ,1,2,3 Jinlu Li,1,4 Jing Yu,1 Ling Wang,4 Xueying Yang,5 Ling Guo ,6 and Shiliang Zhou 1,2 1 State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China 2University of the Chinese Academy of Sciences, Beijing 100043, China 3College of Life Science, Hebei Normal University, Shijiazhuang 050024, China 4Te Department of Landscape Architecture, Northeast Forestry University, Harbin 150040, China 5Key Laboratory of Forensic Genetics, Institute of Forensic Science, Ministry of Public Security, Beijing 100038, China 6Beijing Botanical Garden, Beijing 100093, China Correspondence should be addressed to Ling Guo; [email protected] and Shiliang Zhou; [email protected] Received 21 September 2017; Revised 11 December 2017; Accepted 2 January 2018; Published 19 March 2018 Academic Editor: Fengjie Sun Copyright © 2018 Jiahui Sun et al. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Maleae consists of economically and ecologically important plants. However, there are considerable disputes on generic circumscription due to the lack of a reliable phylogeny at generic level. In this study, molecular phylogeny of 35 generally accepted genera in Maleae is established using 15 chloroplast regions. Gillenia isthemostbasalcladeofMaleae,followedbyKageneckia + Lindleya, Vauquelinia, and a typical radiation clade, the core Maleae, suggesting that the proposal of four subtribes is reasonable. -

Quillaja Saponaria (Molina) Extracts Inhibits in Vitro Piscirickettsia Salmonis Infections
animals Article Quillaja saponaria (Molina) Extracts Inhibits In Vitro Piscirickettsia salmonis Infections Hernán Cañon-Jones 1,* , Hernán Cortes 2, Mario Castillo-Ruiz 3,4 , Trinidad Schlotterbeck 5 and Ricardo San Martín 5 1 Núcleo de Investigación Aplicada en Ciencias Veterinarias y Agronómicas, Facultad de Medicina Veterinaria y Agronomía, Universidad de Las Américas, Santiago 7500975, Chile 2 Desert King Chile, Viña del Mar 2420505, Chile; [email protected] 3 Escuela de Química y Farmacia, Facultad de Medicina, Universidad Andres Bello, Santiago 8370146, Chile; [email protected] 4 Departamento de Ciencias Químicas y Biológicas, Facultad de Ciencias de la Salud, Universidad Bernardo O Higgins, Santiago 8370993, Chile 5 Saponin Research Center, Santiago 7510132, Chile; [email protected] (T.S.); [email protected] (R.S.M.) * Correspondence: [email protected] Received: 15 September 2020; Accepted: 10 November 2020; Published: 3 December 2020 Simple Summary: Bacterial diseases causes massive mortalities in aquaculture and antibiotic use remains the main measure to keep these under control. Pisciricketssia salmonis, an intracellular bacterium only present in Chile, produces high mortalities in farmed salmon and is currently the main reason for using antimicrobials compared to other salmon-producing countries such as Norway. Environmental and antimicrobial resistance concerns have been raised by the local and global public and society, although no scientific evidence has demonstrated such an impact. Thus, there is a constant search for new alternatives that can complement or reduce the use of antimicrobial in intensive salmon farming. Phytochemicals such as saponins from Quillaja saponaria extracts have been proven to prevent and control diseases in other animal production systems. -

Natural Surfactant Saponin from Tissue of Litsea Glutinosa and Its Alternative Sustainable Production
plants Article Natural Surfactant Saponin from Tissue of Litsea glutinosa and Its Alternative Sustainable Production Jiratchaya Wisetkomolmat 1,2, Ratchuporn Suksathan 3, Ratchadawan Puangpradab 3, Keawalin Kunasakdakul 4, Kittisak Jantanasakulwong 5,6, Pornchai Rachtanapun 5,6 and Sarana Rose Sommano 2,5,7,* 1 Interdisciplinary Program in Biotechnology, Graduate School, Chiang Mai University, Chiang Mai 50200, Thailand; [email protected] 2 Plant Bioactive Compound Laboratory, Department of Plant and Soil Sciences, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand 3 Research and Product Development, Department of Research and Conservation, Queen Sirikit Botanic Garden, The Botanical Garden Organisation, Chiang Mai 50180, Thailand; [email protected] (R.S.); [email protected] (R.P.) 4 Department of Entomology and Plant Pathology, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand; [email protected] 5 Cluster of Agro Bio-Circular-Green Industry (Agro BCG), Chiang Mai University, Chiang Mai 50100, Thailand; [email protected] (K.J.); [email protected] (P.R.) 6 Division of Packaging Technology, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand 7 Innovative Agriculture Research Centre, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand * Correspondence: [email protected]; Tel.: +66-53944040 Received: 8 October 2020; Accepted: 6 November 2020; Published: 9 November 2020 Abstract: In this research, we assessed the detergency properties along with chemical characteristic of the surfactant extracts from the most frequently cited detergent plants in Northern Thailand, namely, Sapindus rarak, Acacia concinna, and Litsea glutinosa. Moreover, as to provide the sustainable option for production of such valuable ingredients, plant tissue culture (PTC) as alternative method for industrial metabolite cultivation was also proposed herein. -

Universidade Federal Do Rio Grande Do Sul Faculdade De Farmácia Programa De Pós-Graduação Em Ciências Farmacêuticas
UNIVERSIDADE FEDERAL DO RIO GRANDE DO SUL FACULDADE DE FARMÁCIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS FARMACÊUTICAS CONSTITUIÇÃO QUÍMICA, AVALIAÇÃO DA ATIVIDADE IMUNOADJUVANTE E ESTUDOS DE PROPAGAÇÃO DE QUILLAJA BRASILIENSIS Juliane Deise Fleck Porto Alegre, 2007 UNIVERSIDADE FEDERAL DO RIO GRANDE DO SUL FACULDADE DE FARMÁCIA PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS FARMACÊUTICAS CONSTITUIÇÃO QUÍMICA, AVALIAÇÃO DA ATIVIDADE IMUNOADJUVANTE E ESTUDOS DE PROPAGAÇÃO DE QUILLAJA BRASILIENSIS Tese apresentada por Juliane Deise Fleck para obtenção do TÍTULO DE DOUTOR em Ciências Farmacêuticas Orientadora: Profa. Dr. Grace Gosmann Co-orientadores: Prof. Dr. Arthur Germano Fett-Neto Prof. Dr. Paulo Michel Roehe Tese apresentada ao Programa de Pós-Graduação em Ciências Farmacêuticas, em nível de Doutorado – Pesquisa e Desenvolvimento de Matérias-Primas Farmacêuticas – da Faculdade de Farmácia da Universidade Federal do Rio Grande do Sul e aprovada em 31.07.2007, pela Banca Examinadora constituída por: Prof. Dr. Amauri Braga Simonetti Universidade Federal do Rio Grande do Sul Prof. Dr. Giancarlo Pasquali Universidade Federal do Rio Grande do Sul Prof. Dr. Jarbas Alves Montanha Universidade Federal do Rio Grande do Sul Prof. Dr. Paulo Mazzafera Universidade de Campinas F593c Fleck, Juliane Deise Constituição química, avaliação da atividade imunoadjuvante e estudos de propagação de quillaja brasiliensis / Fleck, Juliane Deise – Porto Alegre : UFRGS, 2007. - xxii, 121 p.: il. Tese (Doutorado). UFRGS. Faculdade de Farmácia. Programa de Pós- Graduação em Ciências Farmacêuticas. 1. Quillaja brasiliensis : Saponinas. 2. Pau-sabão. 3. Rosaceae. 4. Cromatografia líquida de alta eficiência. 4. Atividade adjuvante. 5 MIcropropagação. I. Gosmann, Grace. II. Fett-Neto, Arthur Germano. III. Roehe, Paulo Michel. IV. Título. -

Contributions to the Solution of Phylogenetic Problem in Fabales
Research Article Bartın University International Journal of Natural and Applied Sciences Araştırma Makalesi JONAS, 2(2): 195-206 e-ISSN: 2667-5048 31 Aralık/December, 2019 CONTRIBUTIONS TO THE SOLUTION OF PHYLOGENETIC PROBLEM IN FABALES Deniz Aygören Uluer1*, Rahma Alshamrani 2 1 Ahi Evran University, Cicekdagi Vocational College, Department of Plant and Animal Production, 40700 Cicekdagi, KIRŞEHIR 2 King Abdulaziz University, Department of Biological Sciences, 21589, JEDDAH Abstract Fabales is a cosmopolitan angiosperm order which consists of four families, Leguminosae (Fabaceae), Polygalaceae, Surianaceae and Quillajaceae. The monophyly of the order is supported strongly by several studies, although interfamilial relationships are still poorly resolved and vary between studies; a situation common in higher level phylogenetic studies of ancient, rapid radiations. In this study, we carried out simulation analyses with previously published matK and rbcL regions. The results of our simulation analyses have shown that Fabales phylogeny can be solved and the 5,000 bp fast-evolving data type may be sufficient to resolve the Fabales phylogeny question. In our simulation analyses, while support increased as the sequence length did (up until a certain point), resolution showed mixed results. Interestingly, the accuracy of the phylogenetic trees did not improve with the increase in sequence length. Therefore, this study sounds a note of caution, with respect to interpreting the results of the “more data” approach, because the results have shown that large datasets can easily support an arbitrary root of Fabales. Keywords: Data type, Fabales, phylogeny, sequence length, simulation. 1. Introduction Fabales Bromhead is a cosmopolitan angiosperm order which consists of four families, Leguminosae (Fabaceae) Juss., Polygalaceae Hoffmanns. -

Universidad Católica De Santa María
UNIVERSIDAD CATÓLICA DE SANTA MARÍA FACULTAD DE CIENCIAS FARMACÉUTICAS, BIOQUÍMICAS Y BIOTECNOLÓGICAS ESCUELA PROFESIONAL DE FARMACIA Y BIOQUÍMICA “Evaluación del efecto antipirético y antiinflamatorio del extracto de hojas de Kageneckia lanceolata (lloque) en animales de experimentación, Arequipa 2016” Tesis presentada por las bachilleres en Farmacia y Bioquímica: CCAHUANA CHURATA, DELIA LARICO SANCHO, IVET ROXANA Para obtener el Título Profesional de Químico- Farmacéutico Asesor: Q.F. Fernando Torres Vela AREQUIPA – PERÚ 2017 II DEDICATORIAS A Dios, por darme la vida, buena salud, la fuerza y el coraje para hacer este sueño realidad y por estar en cada momento de mi vida. A mis padres Santiago y Irma, por su amor, su ejemplo, consejos y valores, por motivarme a seguir adelante y por todo el esfuerzo y sacrificio que permitieron que hoy culmine una gran etapa. A mis hermanos Mirian, Alan, A Mis abuelos (QEPD), por Tatiana y Cosme, por darme la quererme y apoyarme siempre, fortaleza para culminar mis esto también se lo debo a ellos y estudios a todos mis amigas(o) que nos brindaron su apoyo durante todo este proceso, y dándonos sus consejos incondicionalmente. Delia III Dedico y agradezco esta tesis a Dios por darme fuerza, fe y haberme guiado a lo largo de este camino; dándome la A mi padre Aquiles Larico por fortaleza para afrontar los todo el esfuerzo y sacrificio por diversos retos que se me brindarme todo el amor, la presentaron. comprensión el apoyo incondicional y la confianza en cada momento de mi vida todo esto es para ti porque eres un ser maravilloso. -

Combined Phylogenetic Analyses Reveal Interfamilial Relationships and Patterns of floral Evolution in the Eudicot Order Fabales
Cladistics Cladistics 1 (2012) 1–29 10.1111/j.1096-0031.2012.00392.x Combined phylogenetic analyses reveal interfamilial relationships and patterns of floral evolution in the eudicot order Fabales M. Ange´ lica Belloa,b,c,*, Paula J. Rudallb and Julie A. Hawkinsa aSchool of Biological Sciences, Lyle Tower, the University of Reading, Reading, Berkshire RG6 6BX, UK; bJodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3DS, UK; cReal Jardı´n Bota´nico-CSIC, Plaza de Murillo 2, CP 28014 Madrid, Spain Accepted 5 January 2012 Abstract Relationships between the four families placed in the angiosperm order Fabales (Leguminosae, Polygalaceae, Quillajaceae, Surianaceae) were hitherto poorly resolved. We combine published molecular data for the chloroplast regions matK and rbcL with 66 morphological characters surveyed for 73 ingroup and two outgroup species, and use Parsimony and Bayesian approaches to explore matrices with different missing data. All combined analyses using Parsimony recovered the topology Polygalaceae (Leguminosae (Quillajaceae + Surianaceae)). Bayesian analyses with matched morphological and molecular sampling recover the same topology, but analyses based on other data recover a different Bayesian topology: ((Polygalaceae + Leguminosae) (Quillajaceae + Surianaceae)). We explore the evolution of floral characters in the context of the more consistent topology: Polygalaceae (Leguminosae (Quillajaceae + Surianaceae)). This reveals synapomorphies for (Leguminosae (Quillajaceae + Suri- anaceae)) as the presence of free filaments and marginal ⁄ ventral placentation, for (Quillajaceae + Surianaceae) as pentamery and apocarpy, and for Leguminosae the presence of an abaxial median sepal and unicarpellate gynoecium. An octamerous androecium is synapomorphic for Polygalaceae. The development of papilionate flowers, and the evolutionary context in which these phenotypes appeared in Leguminosae and Polygalaceae, shows that the morphologies are convergent rather than synapomorphic within Fabales. -

TESIS EFECTO DE LA ADICIÓN DE AGUA.Image.Marked.Pdf
Universidad de Concepción Facultad de Ciencias Forestales Programa de Magíster en Ciencias Forestales EFECTO DE LA ADICIÓN DE AGUA Y NUTRIENTES EN EL CRECIMIENTO E INTERCAMBIO GASEOSO DE Quillaja saponaria Molina (Quillajaceae) POR NATALIA PAZ NAVARRO FUENZALIDA Tesis presentada a la Facultad de Ciencias Forestales de la Universidad de Concepción para optar al grado académico de Magíster en Ciencias Forestales Profesor Guía: Rafael Rubilar Pons Septiembre 2020 CONCEPCIÓN – CHILE © 2020. NATALIA PAZ NAVARRO FUENZALIDA Se autoriza la reproducción total o parcial, con fines académicos, por cualquier medio o procedimiento, incluyendo la cita bibliográfica del documento. iii TABLA DE CONTENIDO ÍNDICE DE TABLAS _____________________________________________ V ÍNDICE DE ILUSTRACIONES _____________________________________ VI RESUMEN ____________________________________________________ VII ABSTRACT __________________________________________________ VIII I. ANTECEDENTES DE Quillaja saponaria Molina ____________________ 1 INTRODUCCIÓN 1 ANTECEDENTES GENERALES DE QUILLAY 4 Taxonomía 4 Descripción general de la especie 5 Distribución territorial y asociaciones 9 Requerimientos ambientales 9 Estado de conservación 10 Interés económico 10 Normativa para su intervención o manejo 12 Silvicultura 12 ALGUNOS EXPERIMENTOS DE CONDICIONES CONTRASTANTES DE DISPONIBILIDAD HÍDRICA Y NUTRICIONAL REALIZADOS CON QUILLAY 14 Estudios de parámetros morfológicos 14 Estudios de parámetros fisiológicos 21 DISCUSIÓN 25 Efecto de la adición de agua y nutrientes en el crecimiento -

IMXQB-80: a Quillaja Brasiliensis Saponin-Based Nanoadjuvant Enhances Zika Virus Specific Immune Responses in Mice
Vaccine 39 (2021) 571–579 Contents lists available at ScienceDirect Vaccine journal homepage: www.elsevier.com/locate/vaccine IMXQB-80: A Quillaja brasiliensis saponin-based nanoadjuvant enhances Zika virus specific immune responses in mice Samuel Cibulski a,b,1, Thais Fumaco Teixeira b,1, Ana Paula Muterle Varela b,1, Matheus Fabião de Lima a, Gabriela Casanova c, Yuri Mangueira Nascimento d, Josean Fechine Tavares d, Marcelo Sobral da Silva d, ⇑ Patrícia Sesterheim e, Diogo Onofre Souza f, Paulo Michel Roehe b, Fernando Silveira g, a Centro de Biotecnologia – CBiotec, Laboratório de Biotecnologia Celular e Molecular, Universidade Federal da Paraíba, João Pessoa, Paraíba, Brazil b Departamento de Microbiologia Imunologia e Parasitologia, Laboratório de Virologia, Universidade Federal do Rio Grande do Sul, Porto Alegre, Porto Alegre, Rio Grande do Sul, Brazil c Unidad de Microscopía Electrónica de Facultad de Ciencias, Universidad de la República, Montevideo, Uruguay d Institute for Research in Pharmaceutical and Medications, Universidade Federal da Paraíba, João Pessoa, Paraíba, Brazil e Centro de Cardiologia Experimental, Instituto de Cardiologia/Fundação Universitária de Cardiologia, Porto Alegre, RS, Brazil f Departamento de Bioquímica, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil g Departamento de Desarrollo Biotecnológico, Instituto de Higiene, Facultad de Medicina, Universidad de la República (UdelaR), Montevideo, Uruguay article info abstract Article history: Vaccine adjuvants are compounds that enhance/prolong the immune response to a co-administered anti- Received 19 May 2020 gen. Saponins have been widely used as adjuvants for many years in several vaccines – especially for Received in revised form 13 November 2020 intracellular pathogens – including the recent and somewhat revolutionary malaria and shingles vac- Accepted 1 December 2020 cines.